Thermogravimetry
advertisement
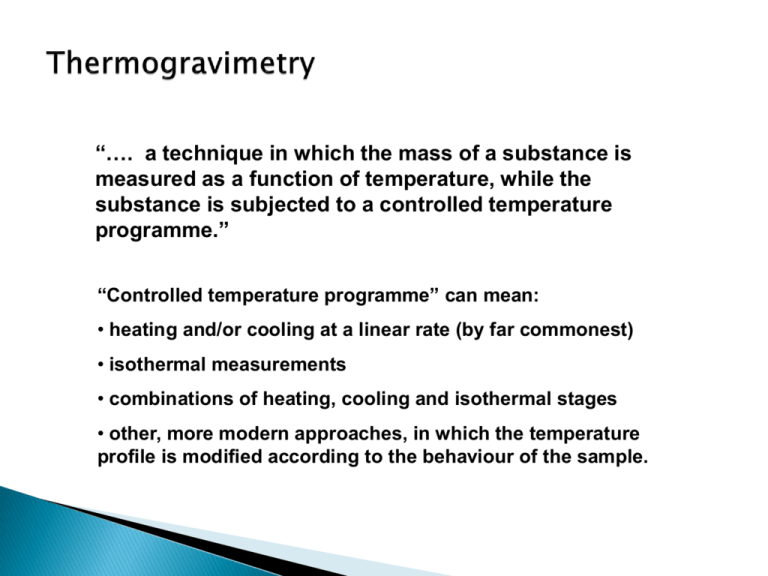
“…. a technique in which the mass of a substance is measured as a function of temperature, while the substance is subjected to a controlled temperature programme.” “Controlled temperature programme” can mean: • heating and/or cooling at a linear rate (by far commonest) • isothermal measurements • combinations of heating, cooling and isothermal stages • other, more modern approaches, in which the temperature profile is modified according to the behaviour of the sample. GAS IN WEIGHT BALANCE CONTROLLER GAS-TIGHT ENCLOSURE SAMPLE HEATER SAMPLE TEMP. POWER FURNACE TEMP. TEMPERATURE PROGRAMMER Mass (%) in green, rate of mass loss (%/°C) in blue. EXCHANGE OF GASES: REACTING GASES IN, PRODUCTS OUT CONVECTION THROUGH SURROUNDING ATMOSPHERE RADIATION FROM FURNACE WALL CONDUCTION THROUGH SAMPLE PAN AND INSTRUMENT INDICATION OF SAMPLE TEMPERATURE A) INSTRUMENTAL B) SAMPLE-RELATED • heating rate • mass • furnace atmosphere and flow-rate • particle size • geometry of pan and furnace • sample history/pre-treatment • material of pan • packing • thermal conductivity • heat of reaction For a given instrument, careful standardisation of experimental procedures leads to highly reproducible results. 10 mg samples of PTFE, heated at 2.5, 5, 10 and 20 °C/min in nitrogen CaC2CO4.H2O in air and nitrogen A) MASS NOISY OR ERRATIC RECORDS • Classical buoyancy CAN ARISE FROM: • Effect temp. on balance • static • convection and/or turbulence • vibration • viscous drag on suspension • pressure pulses in lab. These are lumped together as the “buoyancy” • uneven gas flow correction, and if significant, can be allowed for by a blank run B) TEMPERATURE Temperature calibration difficult to carry out accurately. Many methods exist, but none totally satisfactory. Best accuracy from simultaneous TG-DTA or TG-DSC instrument. a = PVC, b= nylon-6, c = LDPE, d= PTFE PROCESS Ad- or absorption WEIGHT GAIN WEIGHT LOSS Desorption, drying Dehydration, desolvation Sublimation Vaporisation Decomposition Solid-solid reactions (some) Solid-gas reactions Magnetic transitions D. M. Price, D. J. Hourston & F. Dumont, “Thermogravimetry of Polymers”, R. A. Meyers (Ed.), Encyclopedia of Analytical Chemistry, John Wiley & Sons Ltd., Chichester (2000) pp. 8094-8105. G. R. Heal, “Thermogravimetry & Derivative Thermogravimetry”, in P.J. Haines (ed.) Principles of Thermal Analysis & Calorimetry, ch. 4, Royal Society of Chemistry, Cambridge (2002) pp. 10-54. C. M. Earnest (Ed.), Compostional Analysis by Thermogravimetry, ASTM STP 97, American Society for Testing and Materials (1988).









