Lesson 3: Gases and Temperature Changes
advertisement

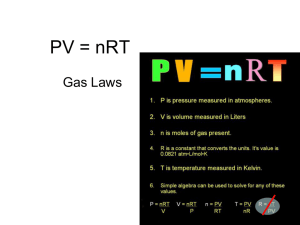
Lesson 3: Gases and Temperature Changes Gas Volume and Temperature - In the 1700s, ballooning was soo popular. Two scientists, Jacques Charles and Joseph Louis Gay-Lassac were especially interested in ballooning. Both men discovered that the volume of a gas was proportional to the temperature. Graphically: Furthermore, the graph, if extrapolated back to zero volume, showed that no matter the starting pressure and volume of a system, at zero volume, the temperature of the gas was always predicted to be -273.15OC. this value is the lowest possible temperature! It is called Absolute Zero, and the value is given as 0 Kelvin, in the Kelvin temperature scale. To convert Celcius to Kelvin +-273.15O To convert Kelvin to Celcius - -273.15O Charles’s Law The relationship between temperature and volume (at constant pressure and n) is called Charles’s law. V is directly proportional to T V1/T1 = V2/T2 Gay-Lassac’s Law The relationship between temperature and pressure (at constant V and n) is called Gay-Lussac’s Law P is directly proportional to T P1/T1 = P2/T2 Learning Intention 4. determine, through inquiry, the quantitative and graphical relationships between the pressure, volume, and temperature of a gas 5. solve quantitative problems by performing calculations based on Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, Dalton’s law of partial pressures, and the ideal gas law 6. Explain Dalton’s law of partial pressures, Boyle’s law, Charles’s law, Gay-Lussac’s law, the combined gas law, and the ideal gas law Uni/Multistructural Relational Extended Abstract State Charles’s and Gay-Lussac’s law mathematically, in words and graphically Define absolute zero Convert between Celcius and Kelvin Solve questions using the Laws Predict the method to perform an inquiry on either law Use the KMT to explain these gas laws Hypothesize how your knowledge of Gay-Lussac’s law could influence how to safely store compressed gas cylinders Squash balls need to be ‘warmed up’ by rallying before beginning a game, comment on the gas laws involved Success Criteria Uni/Multistructural Relational Extended Abstract I can State Charles’s and Gay-Lussac’s law mathematically, in words and graphically I can Define absolute zero I can Convert between Celcius and Kelvin I can Solve questions using the Laws I can Predict the method to perform an inquiry on either law I can Use the KMT to explain these gas laws I can Hypothesize how your knowledge of Gay-Lussac’s law could influence how to safely store compressed gas cylinders I can Squash balls need to be ‘warmed up’ by rallying before beginning a game, comment on the gas laws involved