Charles's Law
advertisement

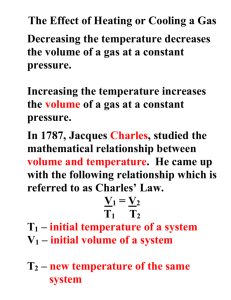
Gases & Atmospheric Chemistry Charles’ & Gay-Lussac’s Gas Laws Unit 5 Review What is the relationship between pressure and volume? Can you tell me the name of the Law for this phenomenon? Who can come and write the Law on the board? Let’s Practice! Kelvin Temperature Scale Absolute Zero = believed to be the lowest possible temperature = -273ºC Temperature at which a volume of gas would be zero 0 K = - 273 oC ( -273.15 oC to be more precise) Kelvin Temperature Scale = a temperature scale with zero kelvin (0 K) at absolute zero and the same size divisions as the Celsius temperature scale Lord Kelvin used Charles’ Law to determine this Kelvin Scale To convert: TK = ToC + 273 e.g. 1. Convert to Kelvin scale a) 25 oC b) - 58 oC 2. Convert to Celsius scale a) 475 K b) 17 K 100 0C 373 K 0 0C 273 K -273 0C 0K Absolute Zero As temperature decreases the volume of a gas decreases (or the pressure drops, if you keep the volume the same) We can deduce how cold you would have to make the gas, in order for the volume to be zero (-273°C or 0K) A gas cannot have a zero volume therefore absolute zero is an unattainable limit Relationship: Volume & Temp. Regardless of gas,V = 0 when T= - 273 oC Charles’s Law Relationship: Volume & Temp. As temperature increases volume increases V T (direct proportion) As temperature increases volume increases Charles’s Law Relationship: Volume & Temp. Charles’s Law = the volume of a gas varies directly with its temperature in kelvin, if the pressure and the amount of gas are constant v = kT v = volume (L) T = Temperature in Kelvin (K) k = constant (slope of the straight line in the graph) Charles’s Law Relationship: Volume & Temp. Charles’s Law can be written comparing any two sets of volumes and temperatures: k = v1/T1 and k = v2/T2 Therefore: v1/T1 = v2/T2 (Charles’s Law) Charles’ Law Sample Problems: 1. 75 mL of dry hydrogen gas at 22 oC is drawn into a syringe. If the temperature is raised to 125 oC, keeping the pressure constant, what volume will this same mass of hydrogen gas occupy? (ans: 1.0 x 102 mL) 2. A balloon filled with dry He(g) has a volume of 3.75 L at 25 oC. The same balloon is placed in a freezer and the volume of the balloon now is 3.05 L. Calculate the temperature (in oC) of the freezer. (ans: -31 oC) Practical Applications Should you throw an aerosol can into a fire? What could happen? When should your automobile tire pressure be checked? Gay-Lussac’s Law Relationship: Pressure & Temp. Graphically: P T (direct proportion) Gay-Lussac’s Law Relationship: Pressure & Temp. Gay-Lussac’s Law Relationship: Pressure & Temp. Pressure & Temperature Law = the pressure exerted by a gas varies directly with the absolute temperature if the volume and the amount of gas remain constant p1/T1 = p2/T2 Gay-Lussac’s Law Sample Problems: 1. Assuming that room temperature is 22 oC, to what temperature must a given mass of gas be raised such that its pressure is doubled? (ans: 317 oC) 2.The pressure gauge reading on a cylinder of oxygen gas is 8.5x103 kPa, at 22 oC. What will be the pressure if the cylinder is placed in boiling water? (ans: 1.1 x 104 kPa) Summary • • • • Boyle’s Law (PiVi=PfVf) o P↑V↓ or P↓V↑ Charles Law (Vi/Ti=Vf/Tf) o V↑T↑ or V↓T↓ Gay-Lussac’s Law (Pi/Ti=Pf/Tf) o P↑T↑ or T↓T↓ Eventually leads to combined gas law o (PiVi/Ti=PfVf/Tf) next chapter.