TURQUOISE-I: SAFETY AND EFFICACY OF ABT
advertisement
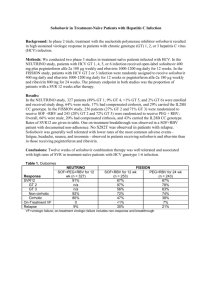
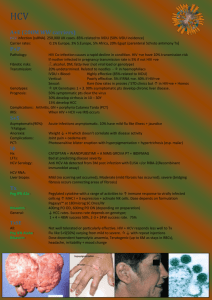
TURQUOISE-I: SAFETY AND EFFICACY OF ABT-450/R/OMBITASVIR, DASABUVIR, AND RIBAVIRIN IN PATIENTS CO-INFECTED WITH HEPATITIS C AND HIV-1 Mark S. Sulkowski, Joseph J. Eron, David Wyles, Roger Trinh, Jay Lalezari, Jihad Slim, Joseph Gathe, Peter J. Ruane, Chia Wang, Richard Elion, Fritz Bredeek, Robert Brennan, Gary Blick, Amit Khatri, Krystal Gibbons, Yiran B. Hu, Linda Fredrick, Tami Pilot-Matias, Barbara Da Silva-Tillmann, Barbara McGovern, Andrew L. Campbell, Thomas Podsadecki 20th International AIDS Conference • Melbourne, Australia • 21 July 2014 Disclosures MS Sulkowski: Consultant/Advisory Board: AbbVie, BMS, Gilead, Idenix Pharmaceuticals, Janssen, Merck, Tobira Therapeutics; Data Safety Monitoring Board: Gilead (funds paid to Johns Hopkins University); Study Steering Committee: Pfizer; Grant/Research support: AbbVie, Boehringer Ingelheim, BMS, Gilead, Merck, Janssen, Vertex Pharmaceuticals (funds paid to Johns Hopkins University). JJ Eron: Grant/Research support: AbbVie, Merck, BMS, GSK/ViiV; Consultant; AbbVie, Gilead, BMS, GSK/ViiV, Merck, Janssen. D Wyles: Grant/Research support: AbbVie, BMS, Gilead, Merck, Vertex Pharmaceuticals; Consultant/Advisor: AbbVie, BMS, Gilead, Janssen. J Lalezari: Research support: AbbVie. J Slim: Speaker Bureau: AbbVie, BMS, Gilead, Merck, Janssen, Genentech. J Gathe: Grant/Research support: AbbVie, Tobira, Boehringer Ingelheim, BMS, GSK, Parexel, Gilead, Huesped. PJ Ruane: Grant/Research support: AbbVie, BMS, Gilead, Merck, Idenix, ViiV, Janssen; Consultant/Advisor: AbbVie, Merck, Gilead: Speaker: Gilead, ViiV, Merck. C Wang: Nothing to disclose. R Elion: Grant/Research support: AbbVie, Merck, BMS, GSK/ViiV, Gilead; Consultant: Gilead, BMS, GSK, ViiV; Speakers bureau: BMS, Merck, ViiV, Gilead, Janssen. F Bredeek: Grant/Research support: AbbVie, BMS, Gilead, Merck, Sumagen, ViiV; Consultant/Advisor: AbbVie, Merck, ViiV. R Brennan: Grant/Research support: AbbVie, Pfizer, Cubist Pharmaceuticals Inc, Achillion, Sanofi Pasteur, ViiV, GSK. G Blick: Grant/Research support: AbbVie, Gilead Sciences, Pfizer, Sangamo Biosciences, ViiV; Consultant/Advisor: BMS, Merck, Serono, ViiV; Speaker: AbbVie, Auxilium, BMS, Merck, Serono, ViiV. R Trinh, A Khatri, K Gibbons, YB Hu, L Fredrick, T Pilot-Matias, B Da Silva-Tillmann, B McGovern, AL Campbell, and T Podsadecki: Employees of AbbVie and may hold stock or options. The design, study conduct, analysis, and financial support of the clinical trials were provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the presentation. All authors had access to all relevant data. This presentation contains information on the investigational products ABT-450/r, ombitasvir (ABT-267), and dasabuvir (ABT-333) and investigational use of ribavirin. TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 2 Background Coinfection with HCV occurs in 20 – 40% of persons living with HIV HCV/HIV coinfection is associated with more rapid liver disease progression2 Liver-related disease is a leading cause of morbidity and mortality in HCV/HIV coinfected patients in the era of ART1 The addition of direct-acting antivirals to pegIFN/ribavirin (RBV) has improved sustained virologic response (SVR) rates, but these regimens are associated with the well-known treatment-limiting toxicities of IFNbased therapy3-5 IFN-free regimens have shown promise with higher SVR rates and an improved safety profile 1Kitahata MM, et al. N Engl J Med. 2009;360:1815-26. 2Martin-Carbonero L, et al. Clin Infect Dis. 2004;38:128-33. MS, et al. Lancet Infect Dis. 2013;13:597-605. 4Sulkowski MS, et al. Ann Intern Med. 2013;159:86-96. 5Rodriguez-Torres M, et al. IDWeek 2013. 3Sulkowski TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 3 3 Direct-Acting Antiviral Regimen (3D) The multi-targeted 3D regimen includes: • ABT-450, a potent NS3/4A protease inhibitor (identified by AbbVie and Enanta) co-dosed with low-dose ritonavir* (ABT-450/r) to increase the peak, trough, and overall drug exposures of ABT-450, enabling once daily dosing6 • Ombitasvir (ABT-267), a potent NS5A inhibitor • Dasabuvir (ABT-333), a non-nucleoside NS5B RNA polymerase inhibitor ABT-450, ritonavir, and ombitasvir are co-formulated as a single tablet Extensive phase 1 drug-drug interaction studies in healthy volunteers with tenofovir, emtricitabine, atazanavir, and raltegravir indicated no clinically meaningful alterations in HCV or HIV drug exposures *Ritonavir does not have antiviral activity against HCV. 6Menon R, et al. HepDART 2009. TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 4 3 Direct-Acting Antiviral Regimen (3D) The 3D regimen, with and without RBV, has been studied in >2700 patients to date High SVR12 rates were achieved with 3D + RBV in genotype (GT) 1infected patients within a phase 3 program • ≥96% SVR12 in treatment-naïve and -experienced patients after 12 weeks of treatment7-10 • 92 – 96% SVR12 in treatment-naïve and -experienced patients with cirrhosis treated for 12 or 24 weeks11 TURQUOISE-I is a 2-part, multicenter, phase 2/3 study assessing the safety and efficacy of 3D + RBV in HCV GT1/HIV-1 coinfected patients, including those with cirrhosis 7Feld 8Ferenci 9Zeuzem 10Andreone JJ, et al. N Engl J Med. 2014;370:1594-603. S, et al. N Engl J Med. 2014;370:1604-1614. 11Poordad F, et al. N Engl J Med. 014;370:1973-82. P, Bernstein D, et al. N Engl J Med. 2014;370:1983-92. P, et al. Gastroenterology. 2014;doi:10.1053/j.gastro.2014.04.045. TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 5 TURQUOISE-I: Part 1 Study Design (N = 63) Open-label Treatment 3D + RBV (N = 31) All patients will be followed for 48 weeks after HCV treatment end SVR12 SVR4 3D + RBV (N = 32) Day 1 Week 12 Week 24 Week 36 3D: coformulated ABT-450/r/ombitasvir, 150 mg/100 mg/25 mg QD; dasabuvir, 250 mg BID RBV: 1000 or 1200 mg daily according to body weight in 2 divided doses (<75 kg and ≥75 kg, respectively) TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 6 TURQUOISE-I: Study Analyses Efficacy analyses • Rapid virologic response (RVR; week 4 of treatment) • End-of-treatment response (EOTR; week 12 or 24 of treatment) • Sustained virologic response (SVR4 and SVR12; post-treatment week 4 and 12, respectively) • On-treatment HCV virologic failure • Post-treatment HCV viral relapse Safety analyses • Maintenance of plasma HIV-1 RNA suppression • Treatment-emergent adverse events • On-treatment lab abnormalities Virologic response is defined as HCV RNA <LLOQ (25 IU/mL) TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 7 TURQUOISE-I: Key Eligibility Criteria • • • • 18 to 70 years of age BMI ≥18 and <38 kg/m2 HCV GT1 infection (plasma HCV RNA >10,000 IU/mL) HCV treatment-naïve or pegIFN/RBV-experienced – For pegIFN/RBV-experienced, prior: Relapse*, Partial response†, or Null response‡ • Child-Pugh A Cirrhosis • HIV-1 infected – Plasma HIV-1 RNA <40 copies/mL – CD4+ count ≥200 cells/mm3 or CD4+% ≥14% – Stable atazanavir or raltegravir-inclusive ART regimen *Relapse: HCV RNA undetectable at or after the end of treatment, but with a detectable level within 52 weeks thereafter † Partial response: >2 log IU/mL HCV RNA reduction at treatment week 12 but detectable at end of treatment 10 ‡ Null response: <2 log IU/mL or <1 log IU/mL HCV RNA reduction at treatment week 12 or 4, respectively 10 10 TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 8 TURQUOISE-I: Baseline Demographics and Disease Characteristics Male, n (%) Black, n (%) Age, mean ± SD Cirrhosis, n (%) IL28B non-CC genotype, n (%) HCV genotype 1a, n (%) HCV RNA (log10 IU/mL), mean ± SD Prior pegIFN/RBV experience, n (%) Naïve Relapse Partial response Null response CD4+ T-cell count/mm3, mean ± SD Atazanavir HIV-1 ART regimen Raltegravir HIV-1 ART regimen 12-Week Arm (N = 31) 24-Week Arm (N = 32) 29 (94) 7 (23) 50.9 ± 6.0 6 (19) 26 (84) 27 (87) 6.54 ± 0.57 29 (91) 8 (25) 50.9 ± 8.3 6 (19) 25 (78) 29 (91) 6.60 ± 0.78 20 (65) 1 (3) 5 (16) 5 (16) 633 ± 236 16 (52) 15 (49) 22 (69) 3 (9) 2 (6) 5 (16) 625 ± 296 12 (38) 20 (63) TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 9 3D + RBV Regimen 12-Week Arm TURQUOISE-I Results: ITT Virologic Response Rates 100 100 100 96.8 96.9 31 31 32 32 30 31 31 32 24-Week Arm 93.5 96.9 93.5 % Patients 80 60 40 20 0 RVR EOTR (Week 4) (Week 12 or 24) SVR4 SVR12 TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 10 3D + RBV Regimen 12-Week Arm TURQUOISE-I Results: ITT Virologic Response Rates 100 24-Week Arm 100 100 96.8 96.9 93.5 96.9 93.5 31 31 32 32 30 31 31 32 29 31 31 32 29 31 % Patients 80 60 40 20 0 RVR EOTR (Week 4) (Week 12 or 24) SVR4 SVR12 TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 11 TURQUOISE-I: Reasons for Non-Response Virologic failure occurred in 2 patients; both were prior null responders with HCV genotype 1a infection and had compensated cirrhosis • Each had resistance-associated variants in at least 2 targets at the time of virologic failure not present at baseline HCV IL 28B Regimen Type of Variants at Time of Failure Patient GT GT Duration Virologic Failure NS3 NS5A NS5B 1 1a T/T 12 wks Relapse at PT D168V M28T S556G Week 2 2 1a T/T 24 wks Breakthrough at None Q30R S556G Week 16 1 patient (12-week Arm) withdrew consent but had an undetectable HCV RNA at last study visit (week 10) No patient discontinued due to adverse events TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 12 TURQUOISE-I: Treatment-Emergent Adverse Events ≥ 10% Event, n (%) Any AE Serious AE Fatigue Insomnia Nausea Headache Upper respiratory tract infection Pruritus Cough Ocular icterus Diarrhea 12-Week Arm (N = 31) 28 (90.3) 0 18 (58.1) 5 (16.1) 5 (16.1) 6 (19.4) 4 (12.9) 6 (19.4) 2 (6.5) 5 (16.1) 1 (3.2) 24-Week Arm (N = 32) 28 (87.5) 0 12 (37.5) 7 (21.9) 6 (18.8) 4 (12.5) 5 (15.6) 2 (6.3) 5 (15.6) 1 (3.1) 4 (12.5) The majority of adverse events were mild or moderate in severity 5 severe adverse events were reported: insomnia, hypophosphatemia, disseminated herpes zoster, tooth abscess, and vertigo No treatment-emergent serious adverse events were reported TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 13 TURQUOISE-I: On-Treatment Laboratory Abnormalities Parameter, n (%) Hemoglobin <10 g/dL Hemoglobin <8 g/dL Total bilirubin >3X ULN ALT >5X ULN AST >5X ULN 12-Week Arm (N = 31) 4 (12.9) 0 11 (35.5) 0 0 24-Week Arm (N = 32) 3 (9.4) 0 6 (18.8) 0 1 (3.1) 6 patients reduced RBV dose due to hemoglobin declines; all achieved SVR Indirect hyperbilirubinemia was the most common laboratory abnormality • 15/17 (88.2%) patients experiencing grade 3 total bilirubin elevations were receiving atazanavir-inclusive ART 5 patients (2 in the 12-week Arm, and 3 in the 24-week Arm) had a confirmed HIV-1 RNA ≥40 copies/mL (but <200 copies/mL) during the treatment period • All 5 patients achieved plasma HIV-1 RNA re-suppression while maintaining the same HIV-1 ART regimen without 3D + RBV interruption TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 14 TURQUOISE-I: Summary This study evaluated the IFN-free 3D + RBV regimen in HCV treatmentnaïve and –experienced patients coinfected with HIV-1, including those with cirrhosis: • SVR12 rate of 93.5% was achieved with 12 weeks of 3D + RBV • SVR4 rate of 96.9% was achieved with 24 weeks of 3D + RBV • 3D + RBV coadministered with atazanavir or raltegravir ART was well-tolerated with no treatment-emergent serious adverse events and no patient discontinuations due to adverse events These results are consistent with those in HCV GT1-monoinfected populations receiving the 3D + RBV regimen TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 15 TURQUOISE-I: Next Steps A cohort of patients on a stable darunavir-inclusive ART regimen who will receive treatment with the 3D + RBV regimen for 12 weeks (Part 1b) will be evaluated TURQUOISE-I, Part 2 will be conducted globally and will initiate later this year TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 16 Acknowledgements The authors would like to express their gratitude to the patients and their families, investigators, and coordinators who made these studies possible. The authors thank AbbVie employees Christine Collins, Sandra Lovell, Rakesh Tripathi, Martin King, Karmin Robinson-Morgan, Gretja Schnell, Jill Beyer, and Thomas Reisch for contributions to the study. Medical writing support was provided by Douglas E. Dylla, an employee of AbbVie. TURQUOISE-I: Safety and Efficacy of ABT-450/r/Ombitasvir, Dasabuvir, and Ribavirin in Patients Co-Infected with Hepatitis C and HIV-1 | AIDS 2014 | 21 July 2014 17