3.2 Molecules of Life
advertisement

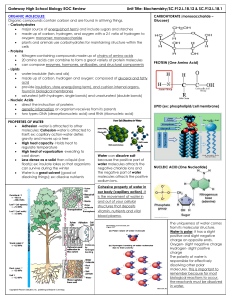
3.2 Molecules of Life 1. 2. 3. 4. Carbohydrates Proteins Lipids Nucleic Acids 3.2 Molecules of Life 1. Carbohydrates •Composed of: – Carbon, oxygen, and hydrogen in a ratio of about – 1 carbon to 2 hydrogen to 1 oxygen •Monomer- monosaccharide •Our main source of energy •Used as structural materials in organisms 3.2 Molecules of Life Carbohydrate # of Monosaccharides Example Monosaccharide Glucose (blood), fructose (fruit), galactose (milk) Disaccharide Sucrose, lactose, maltose Polysaccharide Glycogen(liver + muscle), starch (plants), cellulose (cell walls), chitin (exoskeletons of arthropods) 3.2 Molecules of Life Proteins •Composed of: – Carbon, hydrogen, oxygen, and nitrogen – NO RATIO •Monomer- amino acid •Make up 50% of your dry weight •Functions including structural, defensive, and catalytic roles 3.2 Molecules of Life • Examples: – Enzymes: amylase, catalase, maltase – Structural: collagen – Contractile: actin, myosin (found in muscles) – Transport: hemoglobin (transports oxygen in the blood) – Storage – Hormones: insulin 3.2 Molecules of Life • Amino Acids – Proteins are made up of monomers called amino acids – The sequence of amino acids determines a protein’s shape and function Serine Threonine 3.2 Molecules of Life • Dipeptides and Polypeptides – Two amino acids are joined by peptide bonds to form a dipeptide – A long chain of amino acids is called a polypeptide 3.2 Molecules of Life • Amino acid diagram 3.2 Molecules of Life • Enzymes – Speed up chemical reactions – Bind to specific substrates (aka reactants) – The binding of a substrate with an enzyme causes a change in the enzyme’s shape and reduces the activation energy of the reaction – Typically named for the substrate they work on + “-ase” – The enzyme sucrase works on sucrose – Factors that affect enzymes: heat or pH 3.2 Molecules of Life