Cells 1 Notes (MS Word)
advertisement

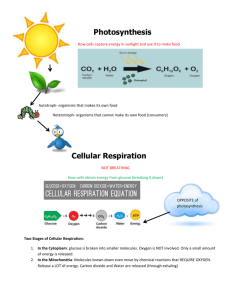
CHEMISTRY OF LIFE A. Inorganic compounds—without carbon 1. Water (H2O)—most important inorganic compound. Many of its unique properties result because water molecules form hydrogen bonds with each other. A hydrogen bond is a weak bond between two molecules resulting from an electrostatic attraction between a proton in one molecule and an electronegative atom in the other. a. b. c. excellent solvent (universal)—because it is polar covalent, other polar molecules and compounds dissolve in it high heat capacity Water molecules are attracted to one another (cohesion) d. 2. Water molecules stick to other polar substances (adhesion) e. Ice floats—when water freezes hydrogen bonds lock water molecules into a crystal structure that has many open spaces Acids and bases—measured on pH scale. Fluctuations in pH can change the rate and nature of internal chemical reactions. a. Acid—strong acids, pH near 0. Characterized by b. c. B. an abundance of H30+ (hydronium) ions. Base—strong bases pH near 14. Characterized by an abundance of OH- (hydroxide) ions. Neutral—pH of 7 (distilled water) Organic compounds—compounds containing carbon (sometimes called biomolecules). Most composed of basic units that repeat. These units are called monomers. Polymers are formed from the condensation of many monomers and are called macromolecules. 1. Carbohydrates—contain carbon, hydrogen, and oxygen (have ratio of 2 hydrogen atoms and 1 oxygen atom per carbon atom). The main source of energy for humans and used as structural compounds. a. Monosaccharides—simple sugars; monomers of carbohydrates. Common monosaccharides include glucose, fructose, and galactose. All have the simple formula of C6H12O6. b. Disaccharides—double sugars. Formed from the condensation of two monosaccharides. Examples include: (1) Maltose (malt sugar)—glucose + glucose (2) (3) c. Polysaccharides—complex sugars. Composed of long chains of monosaccharides. (1) Starch—plant energy storage, polymer of glucose. Forms single line chains of molecules. (2) Glycogen—animal energy storage (in liver and between (3) (4) 2. Lactose (milk sugar)—glucose + galactose Sucrose (table sugar)—glucose + fructose muscle fibers), polymer of glucose. Forms branching chains of molecules. Cellulose—indigestible, dietary fiber for animals. Forms the cell wall of many plant cells. Chitin—makes up the exoskeleton of arthropods and cell walls of fungi. Lipids—also contain carbon, hydrogen, and oxygen, but in a different ratio than carbohydrates. Include fats, oils, waxes, and steroids (fat based hormones). More complex than carbohydrates. Triglycerides (found in fats and oils) are made of glycerol + three fatty acids. More energy per gram than carbohydrates. Used for long term energy storage in humans, cell membranes, and in the nervous system. 3. Proteins—basic building blocks of tissues. Contain carbon, hydrogen, and oxygen as well as nitrogen and sulfur. Made of monomers called amino acids. Used structurally and as biological catalysts called enzymes. a. Amino acids—monomers of proteins; made of an organic acid or carboxyl group (COOH), amino group (NH2), single carbon atom attached to hydrogen, and an R-group. Each of the twenty (20) different amino acid differs in the R-group. b. c. Dipeptides—two amino acids joined by peptide bonds. Polypeptides—three or more amino acids joined by peptide bonds. All proteins consist of these. The sequence of amino acids determines the type of protein. Shapes of protein molecules vary with the sequence of the amino acids and determine their properties. Enzymes—proteins that act as biological catalysts. a. Names of many enzymes end in “ase”. Examples include sucrase (works on sucrose) and maltase (works on maltose). b. c. Each enzyme works on a specific substrate. They lower the amount of activation energy needed to start a chemical reaction, thus speeding up chemical reactions; used over and over again. d. Vitamins may act as co-enzymes. 4. Nucleic acids—carry instructions for cellular activity. a. DNA (deoxyribonucleic acid)—records instructions and transmits them from generation to generation. Found primarily in the nucleus of the cell. b. RNA (ribonucleic acid)—reads and carries out instructions. Found in nucleus and cytoplasm of the cell. c. ATP—high energy compound Both are made of complex monomers called nucleotides CELLS & ENERGY ATP—adenosine tri-phosphate (a type of nucleic acid) 1. 2. 3. 4. Supplies energy for endergonic reactions in organisms Consists of a nitrogen base (adenine), sugar (ribose), and three phosphate groups Energy is stored in bonds between phosphate groups and released for use when bonds are broken Cyclic in nature Photosynthesis—captures energy from light and stores it in the glucose molecule. The simplest formula for photosynthesis is: 6CO2 + 6H2O C6H12O6 + 6O2 Two phases: 1. Light dependent reactions—occur only in light A. B. C. D. 2. Occurs in the grana of the chloroplasts. The electron transport chain which occurs in the thylakoid membranes of the grana produces ATP for use in the light independent reactions. Water molecule split in the electron transport chain occurring in the thylakoid membranes. This process produces hydrogen atoms which are combined with NADP+ forming NADPH to be used in the light independent reactions. Oxygen released into the atmosphere. Light independent reactions (also called the Calvin Cycle)—occur in light or darkness A. B. C. D. Occurs in the stroma of the chloroplasts. Energy from ATP and NADPH produced in the light dependent reactions used in this stage. Glucose is created and stored by plant for later use. Carbon dioxide uptake occurs in this stage. Cell respiration—releases energy from glucose, a total of 38 ATP molecules per molecule of glucose. ATP is adenosine tri-phosphate, a high-energy compound and the only compound that can be used by the body for energy. The formula for cell respiration is: C6H12O6 + 6O2 6CO2 + 6H2O + 38 ATP Two phases of cell respiration: 1. Glycolysis—(anaerobic respiration—w/out oxygen) process that occurs in the cytoplasm of the cell A. B. C. Glucose molecule split into two molecules of pyruvic acid Net production—2 ATP/glucose molecule Fermentation—follows glycolysis if no oxygen is present in the cell. Two types: (1) Alcoholic fermentation—most microorganisms produce ethyl alcohol and carbon dioxide (2) Lactic acid fermentation—most animal cells and some microorganisms produce lactic acid 2. Aerobic respiration—occurs in the mitochondria of eukaryotes if oxygen is present in the cell A. Three parts—net production 36 ATP/glucose molecule (1) (2) (3) B. Pyruvic acid conversion (to acetyl coA)—O ATP/glucose molecule Citric Acid Cycle (Krebs Cycle)—2 ATP/glucose molecule. This cycle turns twice for each molecule of glucose going through cell respiration, therefore one ATP is produced per turn. Electron transport chain (chemical reactions catalyzed by enzymes embedded in the christae of the mitochondria)—34 ATP/glucose molecule 6 molecules of carbon dioxide and 6 molecules of water released TOTAL NET ATP PRODUCTION FOR CELL RESPIRATION—38 ATP/GLUCOSE MOLECULE Electron Transport Chain in Photosynthesis Electron Transport Chain in Cell Respiration