Period 3 Oxides
advertisement

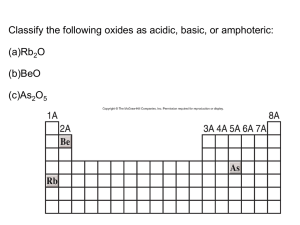
Title: Lesson 4 Period 3 Oxides Learning Objectives: • Understand and explain the trend in acid-base behaviour of the period 3 oxides • Complete an experiment to demonstrate the amphoteric nature of aluminium oxide Refresh Which properties of the alkali metals decrease going down group 1? A. B. C. D. First ionization energy and reactivity Melting point and atomic radius Reactivity and electronegativity First ionization energy and melting point The Period 3 Oxides Element Formula of oxide Structure Reaction of oxide with water Acid/base nature Sodium* Na2O Giant Ionic Na2O + H2O 2NaOH Strongly basic Magnesium* MgO Giant Ionic Slight: MgO + H2O Mg(OH)2 Weakly basic Aluminium Al2O3 Giant Ionic Amphoteric Silicon SiO2 Giant Covalent (Metalloid) Very weakly acidic Phosphorous* P4O10 Molecular Covalent Sulphur* SO2 SO3 Molecular Covalent no direct reaction but: Cl2O7 Molecular Covalent Chlorine Argon P4O10 + 6 H2O 4 H3PO4 Strongly acidic Strongly acidic SO3 + H2O H2SO4 Strongly acidic Cl2O7 + H2O 2 HClO4 no oxides There is a gradual transition from basic to acidic character, reflecting a gradual transition from metallic to non-metallic nature Note: you will only be tested on the elements marked with an asterisk, * 13.3 OXIDES WITH ACIDS & BASES pH OF PERIOD 3 OXIDES: P4O10 SO3 SO2 MgO SiO2 Al2O3 Na2O 13.3 OXIDES WITH ACIDS & BASES A CLASSIC The equation for neutralising an acid with a base is a classic Acid + Base Salt + Water It’s no different for Period 3 oxides You will be expected to write the equations BASIC OXIDES SODIUM & MAGNESIUM: Create 2 other equations for Na & Mg with different acids Na2(s) + H2O(l) 2NaOH (aq) (Alkaline solution formed) These oxides are basic so will neutralise acids. E.g. Sodium oxide reacts with hydrochloric acid to form Sodium chloride and water Na2O(s) + 2HCl(aq) 2NaCl(aq) + H2O(l) E.g. Magnesium oxide reacts with sulphuric acid to form magnesium sulphate and water MgO(s) + H2SO4(aq) MgSO4(aq) + H2O(l) AMPHOTERIC OXIDES ALUMINIUM OXIDE: Aluminium Oxide does not affect pH when added to water because it is insoluble. This is an amphoteric oxide it can react with both acids and alkalis E.g. With sulphuric acid, aluminium sulphate is formed Al2O3(s) + 3H2SO4(aq) Al2(SO4)3 (aq) + 3H2O(l) Reaction with bases: Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium hydroxide. Various aluminates (compounds in which the aluminum is a component in a negative ion) exist, which is possible because aluminum can form covalent bonds with oxygen. E.g. With hot, concentrated sodium hydroxide, sodium aluminate is formed Al2O3(s) + 2NaOH(aq) + 3H2O(l) 2NaAl(OH)4(aq) Acidic Oxides Non metallic oxides react with water to produce acidic solutions: • Phosphorous(V) oxide reacts with water to produce: • Phosphorous (III) oxide reacts with water to produce: • Sulphur trioxide reacts with water to produce sulphuric(VI) acid: • Sulphur dioxide reacts with water to produce sulphuric(IV) acid: ACIDIC OXIDES SILICON, PHOSPHOROUS & SULPHUR: These oxides are all acidic so will neutralise bases P4O10(s) + 12NaOH(aq) 4Na3PO4(aq) + 6H2O(l) SO2(g) + 2NaOH(aq) Na2SO3(aq) + H2O(l) SO3(g) + 2NaOH(aq) Na2SO4(aq) + H2O(l) Complete Test Yourself Questions • Page 103 • Questions 6 – 8 • Check your answers on page 559