Name Date ______ Period _____ Chemistry e
advertisement

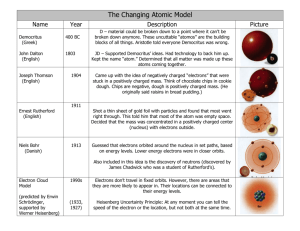
Name ________________________________________________________________ Date __________ Period _____ Chemistry e-Day Lesson 11 1. 2. 3. 4. 5. 6. Print a copy of this page and hand it in when you return to school. go to http://en.wikibooks.org/wiki/High_School_Chemistry The Atomic Theory Further Understanding the Atom Read the lesson Answer the Review Questions, a copy is provided below Review Questions 1. Decide whether each of the following statements is true or false. (a) Cathode rays are positively charged. __________ (b) Cathode rays are rays of light, and thus they have no mass. __________ (c) Cathode rays can be repelled by a negatively charged metal plate. __________ (d) J.J. Thomson is credited with the discovery of the electron. __________ (e) Phosphor is a material that glows when struck by cathode rays. __________ 2. Match each observation with the correct conclusion. Circle your answer. (a) Cathode rays are attracted to a positively charged metal plate. – i. Cathode rays are positively charged. – ii. Cathode rays are negatively charged. – iii. Cathode rays have no charge. (b) Electrons have a negative charge. – i. atoms must be negatively charged. – ii. atoms must be positively charged. – iii. atoms must also contain positive subatomic material. (c) Alpha particles fired at a thin gold foil are occasionally scattered back in the direction that they came from – i. the positive material in an atom is spread throughout like the “batter” in pudding – ii. atoms contain neutrons – iii. the positive charge in an atom is concentrated in a small area at the center of the atom. 3. Alpha particles are: (a) Helium atoms that have extra electrons. (b) Hydrogen atoms that have extra electrons. (c) Hydrogen atoms that have no electrons. (d) Electrons. (e) Helium atoms that have lost their electrons. (f) Neutral helium atoms. 4. What is the name given to the tiny clump of positive material at the center of an atom? 1 http://en.wikibooks.org/wiki/High_School_Chemistry/Further_Understanding_of_the_Atom, Accessed September 30, 2012 David Scott, 2012 Name ________________________________________________________________ Date __________ Period _____ 5. Choose the correct statement. (a) Ernest Rutherford discovered the atomic nucleus by performing experiments with aluminum foil. (b) Ernest Rutherford discovered the atomic nucleus using a cathode ray tube. (c) When alpha particles are fired at a thin gold foil, they never go through. (d) Ernest Rutherford proved that the "plum pudding model" was incorrect. (e) Ernest Rutherford experimented by firing cathode rays at gold foil. 6. Answer the following questions: (a) Will the charges +2 and −2 cancel each other out? (b) Will the charges +2 and −1 cancel each other out? (c) Will the charges +1 and +1 cancel each other out? (d) Will the charges −1 and +3 cancel each other out? (e) Will the charges +9 and −9 cancel each other out? __________ __________ __________ __________ __________ 7. Describe the effect of electrically charged plates on electrons. Electrons are ________________________ negatively charged metals plates and _______________________________ positively charged metal plates? 8. What was J. J. Thomson's name for electrons? ____________________________________________________ 9. A "sodium cation" is a sodium atom that has lost one of its electrons. Circle the best answer in each of the statements below. a. Would the charge on a sodium cation be positive, negative or neutral? b. Would sodium cations be attracted to a negative metal plate, or a positive metal plate? c. Would electrons be attracted to or repelled from sodium cations? 10. Suppose you have a cathode ray tube coated with phosphor so that you can see where on the tube the cathode ray hits by looking for the glowing spot. What will happen to the position of this glowing spot if: (a) a negatively charged metal plate is placed above the cathode ray tube (b) a negatively charged metal plate is placed to the right of the cathode ray tube (c) a positively charged metal plate is placed to the right of the cathode ray tube (d) a negatively charged metal plate is placed above the cathode ray tube, and a positively charged metal plate is placed to the left of the cathode ray tube (e) a positively charged metal plate is placed below the cathode ray tube, and a positively charged metal plate is also placed to the left of the cathode ray tube. David Scott, 2012