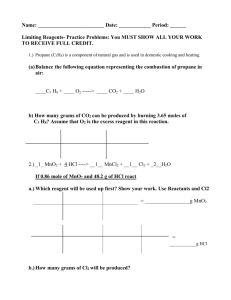
Limiting Reagent

Limiting Reagent
1. Limits or determines the amount of product that can be formed
2. The reagent that is not used up is therefore the excess reagent
These types of problems require 2 sets of tracks. Quantities of both reagents will be given. Therefore, you need to find out which one is the limiting reagent.
Limiting Reagent
One track to determine limiting reagent
A second track to determine product
Limiting Reagent Example problem
How many grams of copper (I) Sulfide can be produced when 80.0 grams of Cu reacts with 25.0 grams of sulfur?
2Cu + S --> Cu
2
S
Pick a reactant and calculate how much of the other reactant is needed.
80.0g Cu 1mol Cu 1mol S 32.1g S
63.5g Cu 2mol Cu 1mol S = 20.2g S
So, 20.2 g of S is needed; 25.0g is supplied
Plenty of S; therefore, Cu is limiting reagent.
Use Cu to solve the problem
80.0g Cu 1mol Cu 1mol Cu
2
S 159.1g Cu
2
S
63.5g Cu 2mol Cu 1mol Cu
2
S
= 1.00x10
2 g Cu
2
S
Limiting Reagent Example Problem - Your Turn
How many grams of hydrogen can be produced when
5.00g of Mg is added to 6.00 g of HCl?
Mg + 2 HCl --> MgCl
2
+ H
2
Pick a reactant and calculate how much of the other reactant is needed.
5.00g Mg 1mol Mg 2mol HCl 36.5g HCl
24.3g Mg 1 mol Mg 1mol HCl = 15.0g HCl
Need 15.0g HCl; have 6.00 g HCl
Not enough HCl; therefore, HCl is limiting reagent
Use HCl to solve the problem
6.00g HCl 1mol HCl 1mol H
2
2.0g H
2
36.5g HCl 2mol HCl 1mol H
2
= 0.164 g H
2
Limiting Reagent Example problem- Your Turn
Acetylene (C
2
H
2
) will burn in the presence of oxygen. How many grams of water can be produced by the reaction of
2.40 mol of acetylene with 7.4 mol of oxygen?
2 C
2
H
2
+ 5 O
2
--> 4 CO
2
+ 2 H
2
O
Pick a reactant and calculate how much of the other reactant is needed
2.40 mol C
2
H
2
5 mol O
2
2 mol C
2
H
2
Need 6.00mol O
2
; have 7.4mol O
2
Plenty of O
2
; so, C
2
H
2 is L.R.
= 6.00 mol O
2
2.4mol C
2
H
2
2mol H
2
O 18.0g H
2mol C
2
H
2
2
O
1mol H
2
O2
= 43.2 g H
2
O