Large Biological Molecules
advertisement

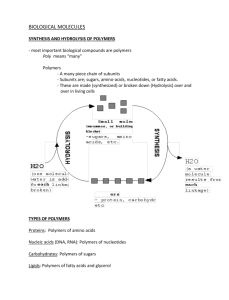
LARGE BIOLOGICAL MOLECULES Chapter 5 FUEL FOR LIVING SYSTEMS Large molecules are important for the basic processes of life Grouped into 4 classes of organic compounds Carbohydrates* Lipids Proteins* Nucleic acids* Important to know how these are made, stored, and destroyed Also, structure and function * are considered macromolecules POLYMERS Chain of similar repeating units linked by covalent bonds E.g CAT-CAT-CAT-CAT=CAT-CAT or the alphabet Carbs, proteins, and nucleic acids are examples The similar repeating units are called monomers E.g CAT or any letter of alphabet Joined and broken by reversible reactions Enzymes can speed the reaction E.g digestion: cells need organic molecules broken down so can be absorbed after which they can be rebuilt POLYMERS Making polymers Breaking polymers Dehydration reaction Links monomers Loss of water for each monomer added Forms a covalent bond 1 2 Hydrolysis reaction Breaks polymers Addition of water for each broken bond 4 3 1 1 2 3 2 3 4 4 1 2 3 4 EXAMPLES OF POLYMERS Small molecules are ordered to dictate life DNA is a polymer composed of 4 monomers (nucleiotides) Creates variation based on arrangement Proteins are polymers from 20 different amino acids (AA’s) Sequence variation separates humans from flowers and individuals from individuals CARBOHYDRATES Simple sugars and polymers of simple sugars Sugars are broken down based on the number of polymers Monosaccharides Disaccharides Polysaccharides Each is joined by a dehydration reaction Polymers of sugar are actually what is generally considered a carbohydrate or starchy food MONOSACCHARIDES Glucose is most common Major nutrient for cells Respiration, fuel for cellular work, and raw material Trademarks of sugars Molecular repeating unit of CH2O Carbonyl and hydroxyl functional groups 3-7 carbons long Hexoses (6 carbons, e.g glucose and fructose) Pentoses (5 carbons, e.g ribose and dioxyribose) End in “-ose” GLUCOSE VS FRUCTOSE Also are examples of what? DISACCHARIDES 2 monosaccharides joined by a covalent bond Result of dehydration reaction Form a glycosidic bond/linkage Maltose glucose + glucose Whoppers, malts, beer Sucrose Glucose + fructose Table sugar Plant sap Lactose galactose + glucose POLYSACCHARIDES Multiple glycosidic linkages Storage material until needed Hydrolysis will break apart to provide sugars to cells Building materials for cell protections 4 types Starch Glycogen Cellulose Chitin POLYSACCHARIDES FOR STORAGE Starch Polymer of many glucose monomers Plants use as storage Form of plastids Stockpiled glucose = stored E E.g potatoes, grains, wheat, and corn Glycogen More branched polymer of glucose Vertebrate storage in liver and muscles Hydrolyzed when sugar is needed Not good for long term because depleted quickly CELLULOSE Cell wall of plant cells Most abundant organic compound on Earth Polymer of glucose with different linkages Straight molecule, grouped to form microfibrils = strong Major component of paper and only of cotton Most animals can’t hydrolyze Undigested, stimulates GI tract through abrasion to stimulate mucous secretion Most fresh fruits, vegetables, and whole grains Insoluble fiber on packages CHITIN Composes arthropod exoskeletons CaCO3 covers body and hardens Molted off and commonly eaten as Ca2+ source Cell walls in fungi Used for surgical thread Dissolvable stitches LIPIDS ‘Grab bag’ of molecules Not true polymers Not really big enough to be macromolecules All mix poorly with water due to hydrophobic nature (hydrocarbon chains) Form ester linkages 3 types Fats Phospholipids Steroids FATS Glycerol (alcohol w/ 3 carbons) and fatty acids (16-18 carbons and carboxyl end) Hydroxyl and carboxyl linkage = ester linkage (triglyceride) Can be saturated or unsaturated Hydrogenated vegetable oils Unsaturated synthetically to saturated by adding hydrogens Peanut butter and margarine to prevent separation Trans fats when conversion changes conformation of double bond Necessary for energy storage (hydrogen bonds) More compact, better for mobility Adipose storage Cushions vital organs and insulates SATURATED VERSUS UNSATURATED CHAINS Saturated All single bonds with H Most animal fats Solid, close bonds; e.g butter Unsaturated Carbon carbon double bonds Most plant and fish fats Liquid, can’t bind close = bend; e.g olive oil PHOSPHOLIPIDS Makes up cell membranes Glycerol with 2 FA’s and 1 phosphate (negative charge) Hydrocarbons make hydrophobic (form tails) Phosphate and attachment are hydrophilic (form heads) Bi-layered to protect hydrophobic from water STEROIDS Lipids with 4 fused rings Synthesized from cholesterol, common in animal cell membranes Precursor to sex hormones Synthetic variants Anabolic steroids (Testosterone) PROTEINS Necessary for almost anything living organisms do Know types and functions from table 5.1 Enzymes regulate metabolism by acting as catalysts Speed reactions w/o being consumed Unique 3D shapes Formed from polypeptides (polymers of amino acids) 20 AA’s, same set for all Protein = 1+ polypeptide folded and coiled into specific 3D shape AMINO ACID MONOMERS Common structure Carboxyl and amino group α-carbon is middle with H and R group (variable) Determines specific AA from fig. 5.17 Side chains grouped by properties Nonpolar, hydrophobic Polar, hydrophilic Acidic, (-) charge b/c carboxyl group Basic, (+) charge b/c amino group Charges = hydrophilic Polymers formed by peptide bonds STRUCTURE AND FUNCTION Polypeptides ≠ protein AA sequence does 4 levels of structure 1°-seq of AA, determined by genes 2°-repeated coils or folds for overall shape H-bonds b/w carboxyl and amino backbone α-helix = H bonds b/w 4th AA ß-pleated sheet = 2+ regions of H bonds 3 °- interactions b/w side chains Hydrophobic interaction = side chains cluster in Disulfide bridges = -SH side chain interactions 4°-overall structure of 2+ polypeptides PROTEIN STRUCTURE AND FUNCTION Polypeptides ≠ protein 1°: genes decide 2°: H-bonds b/w carboxyl and amino α-helix: 4th AA Β-sheet: 2+ regions of side by side H-bonds 3°: hydrophobic side chains and disulfide bridges 4 : 2+ polypeptides CHANGING PROTEIN STRUCTURE Sickle cell Single AA substitution in hemoglobin Abnormal shape RBC’s that clogs vessels Denaturation Proteins unravel and lose shape pH, [salt], temp, and other effects can cause Inactivates proteins Removing agents might reverse Misfolding Accumulate and cause detrimental problems E.g Alzheimer’s and Parkinson’s disease PROTEIN MISFOLDING Often times unfolding exposes hydrophobic areas to the aqueous solutions surrounding the protein Aggregates to protect itself NUCLEIC ACIDS Polymers of nucleotides (polynucleotides) Blueprint for proteins to control all of cellular workings Control of reproduction DNA RNA proteins Central dogma of molecular biology Occurs in ribosomes Monomer is a nucleotide Structure consists of 3 components Nitrogenous base 5 carbon sugar Phosphate group NUCLEOTIDE Nitrogenous base Pyrimidine = a 6 member carbon and nitrogen ring cytosine (C), thymine (T), uracil (U) Purines = 6 member carbon ring fused to a 5 member ring (smaller name, bigger structure) adenine (A) and guanine (G) DNA – C, T, G, and A RNA – C, U, G, and A 5 Carbon sugar Ribose Deoxyribose (missing oxygen) NUCLEOTIDE POLYMERS Phosphodiester linkage = phosphate joins sugars of 2 nucleotides For backbone of DNA Phosphate on 5’ carbon joins hydroxyl on 3’ carbon DNA codes 5’ -3’ Sequence of bases unique to each gene Linear order of nitrogenous bases in a gene specifies AA sequence (which level of structure ?) Start codon ATG and AUG = DNA and RNA Stop codon UAG, UAA, UGA DOUBLE HELIX 1st proposed by Watson and Crick Sugar-phosphate backbones are antiparallel Nitrogenous bases face in and H-bonds hold them together 2 strands are complementary Binding specific A binds w/ T G binds w/ C