Chapter 4
advertisement

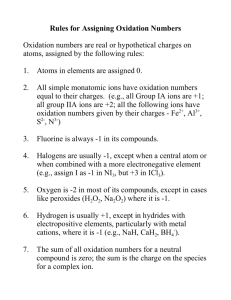
Chemical Quantities and Aqueous Reactions Patterns of Reactivity Five basic types of reactions. 1 . Combination – two substances combine to make one new one. Generic: A + B C Ex) 2 Mg(s) + O2(g) 2 MgO(s) 2. Decomposition – one substance decomposes to several new ones. Generic: A B + C Ex) 2 NaN3(s) 2 Na(s) + 3 N2(g) Patterns of Reactivity 3. Single Replacement – one element replaces the other. Generic: A + BC AC + B Ex) 2 AgNO3(aq) + Cu(s) Cu(NO3)2(aq) + 2 Ag(s) 4. Double Replacement (aka “Metathesis”) – trading partners. Generic: AB + CD AD + CD Ex) Hg(NO3)2(aq) + 2 NaI(aq) HgI2(s) + 2 NaNO3(aq) 5. Combustion – a rapid reaction with O2(g) producing a flame. Ex) CH4(g) + 2 O2(g) CO2(g) + 2 H2O(l) Interpreting a Reaction A simple reaction like: N2(g) + 3 H2(g) 2 NH3(g), can be interpreted on many levels. Molecular Level: one molecule of N2 plus three molecules of H2 react to form two molecules of NH3 Interpreting a Reaction For this reaction, we can establish that: 1 molecule N2 = 3 molecules H2 1 molecule N2 = 2 molecules NH3 3 molecules H2 = 2 molecules NH3 LEP #1 Interpreting a Reaction The molecular level is really not practical as we cannot do reaction on this scale. Rather, we can do them on a mole scale. Thus: one mole of N2 plus three moles of H2 react to produce two moles of NH3. This means our relations can be shortened to moles. LEP #1 Limiting Reactant If given amounts of both reactants, we may run out of one of them first. This reactant limits how much can be made. Analogy: Putting together a bicycle – parts on hand are 200 frames and 350 wheels. How many bicycles can you make? Ex) 2 H2 + O2 2 H2O Suppose a vessel contained 10 molecules of H2 and 7 molecules of O2. How many water molecules are possible? Limiting Reactant This also applies to mole amounts as well. LEP #2 Stoichiometry Pronounced: stoy-key-OM-uh-tree. Relating quantities in chemical reactions – in particular – masses. Cannot use mole-to-mole ratios to convert mass of one substance to mass of another by one single step. A mass-to-mass conversion must be done in three steps. Stoichiometry Stoichiometry Can be used to find a mass of another reactant or a product. Can be part of a limiting reactant where amounts of both reactants are given. Can also be asked to find a percent yield. Percent Yield = Actual Mass Obtained 100 Theoretical Mass Where the Theoretical Mass is the maximum amount possible based on your limiting reactant. LEP #3 and #4 Solution A solution is a homogeneous mixture. Consists of a solute and the solvent. Ex) NaCl added to water Chapter 12 will show us a wide variety of solutions. Chapter 4 – only water is the solvent. Solution Concentration There are many methods for expressing a solution’s concentration and we will see more methods in Chapter 12. Chemists typically use molarity (M). Molarity = Molarity Calculations can be from a mass and volume – LEP #5. Or from a molarity and volume – LEP #6. Or involve a dilution – LEP #7. Solution Stoichiometry Reactions that take place in solution can be analyzed using molarity and volume. Molarity can be used a conversion factor. Can be calculation to find amount of other reactant (volume or mass). Can be limiting reactant problem. LEP #8, #9 Dissolving an Ionic Compound When sodium chloride is added to water, the ions are pulled apart. By itself, pure water is a poor conductor of electricity. When an ionic compound dissolves, it produces ions. These ions can carry a charge through the solution and are referred to as electrolytes. Electrolytes vs. Non-electrolytes Electrolytes Strong Electrolyte – completely dissociates to produce 100% ions in solution. Ionic compounds – must be soluble in water Strong Acids (6) = Strong Bases = Electrolytes Weak Electrolytes – partially ionize to produce a few ions. Solution is weakly conducting. Weak acids – formula starts with “H”, but not on list of S.A. Ex) HC2H3O2 , HF, HCHO2, etc. Weak bases – ammonia or amines. Ex) NH3, CH3NH2, CH3CH2NH2 Non-electrolytes Molecular compounds that dissolve in water, but produce no ions. Sugar molecules, Cn(H2O)n Ex) C6H12O6, C5H10O5 C1 to C4 Alcohols, Aldehydes, Ketones Ex) CH3OH, CH3 C CH3 || O LEP #10 Solubility Rules Provides general solubility – only gives “black or white” details. No detail on extent of solubility. Ex) 34 g / 100mL for KCl Some soluble compounds have low limit. Ex) 0.17 g / 100mL for Ca(OH)2 Some insoluble compounds may be slightly soluble. Ex) 0.45 g / 100mL for PbCl2 Insoluble compound = (s). Soluble compound = (aq). LEP #11, #12 Activity Series Ranks the metals from most reactive to least reactive. A metal HIGHER on the activity series will replace (react) any metal ion beneath it. Will Mg(s) react with Cu+2(aq)? Will Sn(s) react with Fe+2(aq)? Writing a Reaction A molecular equation shows all compounds written as if they were molecules – even substances that are known to exist as ions. An ionic equation shows all aqueous compounds as ions in solution. All aqueous compounds are broken apart. Writing a Reaction AgNO3(aq) + NaCl(aq) A Net Ionic equation removes all of the spectator ions. Spectator ions are ones that do not change from reactants to products. These are the aqueous ions. Single Replacement • Writing a single replacement reaction from scratch. • A + BC • A and B trade places as long as predicted by Activity Series. • Watch out for charges! • Special treatment for H+ (acid) – will generate H2(g)! • LEP #13 Double Replacement • Writing a double replacement reaction involving a • • • • • precipitation from scratch. AB + CD Trade partners – A goes with D and C goes with B. Once again, charges MUST be observed when recombining and formulas are written with cation first. Reaction only happens if one of the two products is Insoluble (s). LEP #14 a, b Double Replacement • These reactions can also produce a gas like CO2 or H2S. • An acid plus a carbonate or bicarbonate • An acid plus any sulfide • An acid and a base react to form water – also called neutralization. • Acid plus a base form water plus a “salt”. • LEP #14 c, d, e Oxidation – Reduction Referred to as “redox” for short. This process involves the loss and gain of electrons. Corrosion of metals Batteries Oxidation = the loss of electrons. Reduction = the gain of electrons. OIL RIG = Oxidation Numbers An accounting method used to assign each element in a compound and oxidation state (number). Rules: 1. The oxidation state of any element in its standard state is zero. Ex) Na(s), Cl2(g), P4(s), etc. 2. The oxidation state of a monoatomic ion is equal to its charge. Ex) Cu+2(aq) = +2, Cl-1(aq) = -1 3. The sum of all oxidation states in a compound should equal zero. Oxidation Numbers 4. The sum of all oxidation numbers in an ion will equal the charge on the ion. 5. Group 1A metals = +1, 2A metal = +2, Al = +3. 6. Halogens: F = -1; Cl, Br, and I are usually -1 unless when O is present. Ex) NaClO 7. O = -2, unless in a peroxide like H2O2. S = -2 unless when O is present. 8. H = +1. LEP #15 Redox Reactions Once all elements in a reaction have been assigned oxidation numbers, inspect to see if two elements have changed. Cannot have oxidation without reduction! Ex) 2 Cu(s) + S(g) Cu2S(s) Ex) Zn(s) + 2 HCl(aq) ZnCl2(aq) + H2(g) LEP #16 Redox Reactions Just to make things a little more confusing… The element or compound that was reduced = oxidizing agent. The element or compound oxidized = reducing agent. Always from the perspective of the REACTANTS. Acid-Base Titration These involve the neutralization reaction. Endpoint = when all of the unknown solution has reacted. Indicator = substance that changes color when the endpoint has been achieved. Acid-base Titration Delivery of the known solution is achieved using a buret. Measures to nearest 0.05mL. Acid-base Titration Typically, the known solution is the base like NaOH. LEP #17 Redox Titration Assay analysis of an ore and many other applications. Common oxidizing agent is KMnO4 LEP #18