Hydration of Alkynes
advertisement

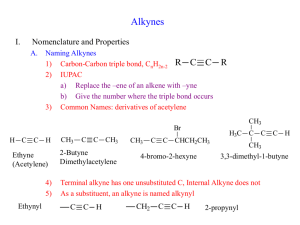
Chapter 9: Alkynes 9.1: Sources of Alkynes (please read) 9.2: Nomenclature Systematic Nomenclature: Prefix-Parent-Suffix Naming Alkynes: Suffix: -yne Many of the same rules for alkenes apply to alkynes 1. Number the carbon chain from the end of the carbon nearest the triple bond 2. The alkyne position is indicated by the number of the alkyne carbon in the chain 3. Compounds with two triple bonds are referred to as diynes, three triple bonds as triynes, etc 9.3: Physical Properties of Alkynes (please read) 205 9.4: Structure and Bonding in Alkynes: sp Hybridization H C C H acetylene (ethyne) bond angles: H-C-C = 180° (linear geometry bond distances: C-H = 106 pm CC = 120 pm Each carbon is sp hybridized – linear geometry CC bond consists of one σ–bond (sp hybridized orbitals) and two π–bond (unhybridized p-orbitals) (see ch. 2 notes) Bond dissociation energies (ΔH°C-C) H° C=C = 611 KJ/mol H° CC = 820 KJ/mol H° C–C = 368 KJ/mol H° C–C = 368 KJ/mol -bond = 243 KJ/mol -bonds = 452 KJ/mol 226 KJ/mol per π -bond The -bond of an alkene is ~17 KJ/mol more stable than the -bond of an alkyne. 206 H H H ~111° 111 pm H H 106 pm C C H H 134 pm H 153 pm H 180° 110 pm C C ~121° C C H 120 pm H H hybridization of C geometry sp3 sp2 sp tetrahedral trigonal planar linear ΔH°C 368 kJ/mol 611 kJ/mol 820 kJ/mol -C ΔH° 410 kJ/mol 452 kJ/mol 536 kJ/mol 25% 33% 50% 62 45 26 C-H % s character pKa 207 9.5: Acidity of Acetylene and Terminal Alkynes In general, the C-H bond of hydrocarbons are very weak acids. The R-CC-H bond of a terminal acetylene is weakly acid, pKa ~26. H H H C H H C H H + H+ pKa = 60 + H+ pKa = 45 + H+ pKa = 26 H H _ H C C H _ C C H H H R C C R C C H _ A strong base can deprotonate terminal acetylenes to generate an acetylide anion. R C C H pKa = 26 + B: _ Na + R C C Na + _ + B:H pKa = 208 9.6: Preparation of Alkynes by Alkylation of Acetylene and Terminal Alkynes - Acetylide anions are strong nucleophiles and will undergo nucleophilic substitution reactions with primary alkyl halides, resulting in the formation of a C-C bond. R C C R _ + H Na + R THF C Br SN2 H + R C C C H NaBr H new C-C bond formed Alkylation of acetylide anions is a general method of making higher alkynes from simpler alkynes. + H2N: Na+ H C C H H C C + H3CH2CH2C-Br H C C H3CH2CH2C C C H pentyne H3CH2CH2C C C H pentyne H3CH2CH2C C C + H2N: Na+ + H3C-I H3CH2CH2C C C H3CH2CH2C C C CH3 2-hexyne 209 Alkylation of acetylide anions is generally limited to primary alkyl halides. Acetylide anions act as a base with secondary and tertiary alkyl halides resulting in E2 elimination. 9.7: Preparation of Alkynes by Elimination Reactions Double dehydrohalogenation reaction R R X2 H X R R NaNH2 H X H vicinal dihalide R R NaNH2 R R X H H R R NaNH2 X X geminal dihalide 3 equivalents of NaNH2 are required for preparing terminal alkynes from from 1,2- or 1,1-dihaloalkane. 210 9.8: Reactions of Alkynes - alkynes undergo addition reactions similar to alkenes. Increasing oxidation state C C C C C C Cl C Cl Cl C Cl C Cl Cl O C OH C O C OR 9.9: Hydrogenation of Alkynes - The hydrogenation of an alkyne cannot be stopped at the alkene stage under normal hydrogenation conditions (H2, metal catalyst) R1 C C R2 H2, Pd/C H H C C R1 R2 cis alkene intermediate H2, Pd/C R1 H H C C R2 H H 211 The π-bonds of alkenes are slightly more reactive toward hydrogenation than the π-bond of an alkene. Lindlar’s catalyst: “poisoned” palladium catalyst Pd on CaCO3 + Pb(OAc)4 + quinoline (amine) “poisons” reduce the catalysts activity so only the most reactive functional groups are hydrogenated H2, Lindlar's Catalyst H R1 C C R2 H C C R1 R2 cis -addition of H2 Hydrognation can be stopped at the cis-alkene stage with Lindlar’s catalyst 9.10: Metal-Ammonia Reduction of Alkynes - Dissolving Metal Reduction: Li(0) metal in liquid ammonia (NH3) e• Li(0) in NH3 R1 C C R2 Li, NH3, (CH3)3COH H (solvated electron) R2 C C R1 H trans-alkene 212 9.11: Addition of Hydrogen Halides to Alkynes Addition of HX to acetylenes: Markovnikov addition X HX R C C H only useful for terminal or symmetrical acetylenes (R1 = R2) C R C H H vinyl halide X HX R1 C C R2 R1 C C R2 H trans addition of HX Mechanism ? HX R C C H R + C C H H vinyl carbocation rate = k [alkyne][HX]2 213 Carbocation Stability R R R R H H H + R C C H 3° alkyl 2° alkyl 1° alkyl 2° vinyl R C+ > R C+ > H C+ ~ H > H C+ H + H C C H ~ H 1° vinyl methyl carbocation gem-dihaloalkanes are obtained with excess HX - addition follows Markovnikov’s rule X HX R1 C C R2 excess R1 C X X R2 C R1 C H H HX C R2 H R2=H or R2=R1 gem-dihaloalkane Anti-Markovnikov addition of HBr to terminal alkynes in the Presence of peroxides (radical mechanism) R C C H HBr peroxides H HX R1 C C H Br R1 C C H Br 214 9.13: Addition of Halogens to Alkynes X X2 R1 C C R2 R1 C C X X R2 C R1 C X X X2 R2 excess X anti addition of X2 9.12: Hydration of Alkynes Alkenes Alkynes hydration Alcohols hydration Ketones or Aldehydes Hg (II) catalyzed hydration of alkynes: similar to oxymercuration - Markovnikov Addition R C C H OH HgSO4, H2SO4, H2O R1 C C H enol H O tautomerization R CH3 methyl ketone 215 Tautomerization - equilibrium between two isomers (tautomer), which differ by the migration of a proton and a -bond. Keto-enol tautomerization - normally favors the keto form (C=O) C=C C-O O-H H° = 611 KJ/mol 380 426 O C H O C C enol C H C=O C-C C-H H° = 735 KJ/mol 368 420 keto Keto-enol tautomerization is acid-catalyzed 216 Hydroboration of Alkynes - anti-Markovnikov hydration of an alkyne (complementary to the Hg(II) catalyzed method) R C C H 1) BH3, THF 2) H2O2, NaOH O R H Aldehyde product Hg(II)-catalyzed hydration and hydroboration are equivalent for internal alkynes 1) BH3, THF 2) H2O2, NaOH R1 C C R2 R1 HgSO4, H2SO4, H2O O O R2 + R1 R2 R1 C C R2 Ketone products Hydration of an internal alkyne is only useful for symmetrical acetylenes (R1 = R2) 217 9.14: Ozonolysis of Alkynes Ozonolysis of alkenes (sect. XX): R1 R2 R3 1) O3 2) Zn R1 R3 O R4 + ketones and aldehydes O R2 R4 Ozonolysis of Alkynes 1) O3 2) Zn R C C H O + R C H2CO3 OH terminal alkyne Carboxylic acid R1 C C R2 1) O3 2) Zn + R1 C OH internal alkyne O O C R2 HO Carboxylic acids Alkynes are less reactive toward ozonolysis than alkenes. An alkenes can be oxidatively cleaved by ozone in the presence 218 of an alkyne. C-C bond forming reactions are important for organic synthesis Prepare octane from 1-pentyne H3CH2CH2C C CH CH3CH2CH2CH2CH2CH2CH2CH3 Prepare (Z)-2-hexene from 1-pentyne H3CH2CH2C C CH Z-2-hexene Prepare 4-octanone from acetylene O HC CH 219