lac
advertisement

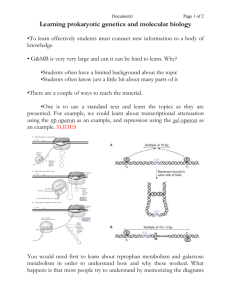
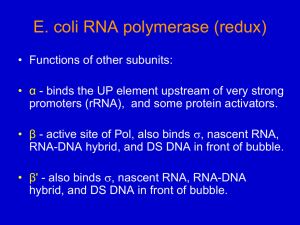
Regulation of Transcription in Prokaryotes •lac Operon •ara Operon Operon: A group of contiguous, coordinately controlled genes. A single mRNA molecule transcribed from an operon contains multiple ORFs - called a polycistronic message. Transcriptional Regulation of Operons -Regulatory sequences adjacent to an operon determine whether it is transcribed or not. -Regulatory sequences are primarily ‘operators’ (repressor binding sequences). Can also include activator binding sequences. -Regulatory proteins work with regulatory sequences to control transcription of the operon. -Regulatory proteins act as activators or repressors Induction and Repression •Induction refers to increased expression of genes in response to a metabolite. •Repression refers to decreased expression of genes in response to a metabolite. •IPTG, the artificial inducer of the lac operon, is an example of a ‘gratuitous’ inducer: It cannot be metabolized by b-galactosidase enzyme, but it still induces b -galactosidase synthesis. The lac operon allows the option of lactose utilization as a carbon source Genes Encoded by the lac Operon •lacZ encodes b -galactosidase that cleaves lactose into galactose and glucose, AND converts lactose to the inducer allolactose. •lacY encodes galactoside permease which is required for transport of lactose into the cells. •lacA encodes galactoside transacetylase which transfers acetyl groups from acetyl-CoA to b -galactosides. The biological function of the enzymatic activity is unclear. b -galactosidase Activity 1. 2. β-galactosidase lactose b -1,4 linkage allolactose b-1,6 linkage Regulation of the lac operon involves: Negative control Positive control Cis-acting DNA sequence Trans-acting protein factors Negative control of the lac operon Monod showed that Z+Y- mutants did not take up a radioactively labeled galactoside in the presence of inducer. He concluded that lacY must encode an enzyme responsible for transporting the galactoside into cells: called it galactoside permease. lac Genetics I lac Genetics II Jacob and Monod’s Operon Hypothesis Based on Genetics 1. There are two key control elements of the operon: the repressor gene and the operator to which the repressor gene product binds. 2. There is a specific interaction between the inducer and repressor that prevents the repressor from binding to the operator. 3. All three lac genes are clustered under a single control unit. 4. Subsequent deletion analysis showed that there is a promoter necessary for the expression of all three lac genes. Biochemical studies confirm all of the tenets of Jacob’s and Monod’s hypothesis. Isolation of the lac Repressor Assay for lac repressor activity: Binding to the labeled synthetic inducer IPTG. Problem: lac repressor is present in very tiny quantities in the cell. Solution: Isolate repressor from mutant E. coli strain with lacIt mutation that causes repressor to bind to IPTG more tightly than normal. Prediction: If the protein isolated really was the lac repressor, then it should bind to the lac operator in an inducer sensitive manner (i.e. the addition of inducer should prevent the repressor from binding to the operator). Experiment: Cohn and colleagues used a nitrocellulose filter binding assay. They mixed 32P-labeled lacO DNA with the protein from above, and either added or left out IPTG. Result: In the presence of protein and ABSENCE of IPTG the labeled lacO DNA was retained on the filter. Conclusion: The lac repressor had been isolated. Also found the Oc operator bound with lower affinity to the repressor as compared to the WT operator. How does the lac repressor work? 1971 - Pastan and colleagues showed that RNA Pol can bind tightly to the lac promoter and form an open promoter complex in the presence of the lac repressor. 1987 - Straney and Crothers showed that RNA Pol and the lac repressor can bind simultaneously to the lac promoter. Hypothesis: The lac repressor prevents the transition from transcription initiation to elongation. Lee and Goldfarb Expt. Expt: Run-off transcription assay. 1. Pre-incubate DNA (contains lac control region and beginning of lacZ gene) with (or without) repressor for 10 min. 2. Add RNA Pol. 3. Add heparin, and all components of transcription reaction except CTP. 4. Add labeled [a-32P]CTP with or without IPTG. Result of Lee and Goldfarb Expt. Conclusion: RNA Pol forms an open complex with the lac promoter even in the presence of the lac repressor in vitro. (sequence: promoter & DNA , repressor + or -, RNA pol, heparin, all rxn components except CTP, then alpha 32P CTP. Observed that short abortive transcripts are even shorter in the presence of the repressor. Added further support for the idea that the repressor prevents the transition from initiation to elongation. Problem with Lee and Goldfarb Expt: Nonphysiological concentrations of repressor, RNA Pol etc. were used. (Concentrations were higher than in vivo.) Record and Colleagues: Kinetic studies of effect of lac repressor on dissociation of RNA Pol from promoter in vitro using conditions closer to in vivo situation. • Made RNA Pol/lac promoter complexes. • Added or didn’t add heparin or lac repressor. • Measured rate of abortive transcript synthesis by including a fluorescently labeled UTP analog. Labeled pyrophosphate was released when this analog was incorporated into RNA. Got results different from those of Lee and Goldfarb! Result of Record and colleagues Expt. Results suggest that the lac repressor does prevent binding of RNA Pol to the promoter! There are 3 lac operators All three operators are important for repression. Structure of lac repressor tetramer bound to DNA one dimer of tetramer operator DNA Positive control of the lac operon CAP + protein These are trans-acting factors. Cis-acting sequence is activator (or CAP) binding site. cAMP signals low glucose activator binding-site lac operon off low lac operon very weakly on Note:the promoter above is WEAK. Binding of CAP (next slide) helps transcription. lac operon fully induced CAP -cAMP Activity •CAP-cAMP complex recruits RNA Pol to the major (P1) promoter of the lac operon to form closed promoter complex CAP and RNA Pol bind cooperatively to DNA, and physically interact. Evidence: 1. CAP and RNA Pol cosediment in presence of cAMP. 2. When CAP and RNA Pol are both bound to lac control region, they can be chemically cross-linked. 3. DNase footprinting shows that CAP and RNA Pol bind to adjacent binding sites in DNA. 4. Certain mutations in CAP that reduce activation specifically affect what is thought to be RNA Pol binding region of CAP. (These same mutations don’t affect binding of DNA by CAP). 5. Deletion of aCTD of RNA Pol, region thought to bind to CAP, prevents activation by cAMP-CAP. 6. In crystal structure, cAMP-CAP and aCTD are in contact when cAMP-CAP and RNA Pol both bound to DNA. Interaction of CAP-cAMP, RNA Pol and DNA of lac control region The ara Operon • Another example of operon that has both positive and negative regulation • The genes araB, A, and D encode the 3 arabinose metabolizing enzymes. • The araC gene encodes the control protein AraC which is both a positive regulator (in the presence of arabinose) and a negative regulator (in the absence of arabinose). • The cAMP-CAP complex also acts as a positive regulator. Organization of the ara operon Control of the ara Operon I - Negative araPBAD • When arabinose is absent, the AraC protein acts as a negative regulator. • AraC acts as a dimer, and causes the DNA to loop. Looping brings the I1 and O2 sites in proximity to one another. • One AraC monomer binds to I1 and a second monomer binds to O2. • Binding of AraC prevents RNA Pol from binding to the PBAD promoter Control of the ara Operon II - Positive araPBAD • When arabinose is present, it binds to AraC and changes AraC conformation. • An arabinose-AraC dimer complex binds preferentially to I1 and I2, and NOT to O2 which causes opening of the loop. This allows RNA Pol to bind to PBAD. • If glucose levels are low, cAMP-CAP complex binds to Pc. • Then active transcription occurs. 39 Negative autoregulation of araC transcription High levels of AraC cause the protein to bind to O1 and inhibit transcription of the araC gene negative autoregulation.