Slajd 1 - InterQuality
advertisement

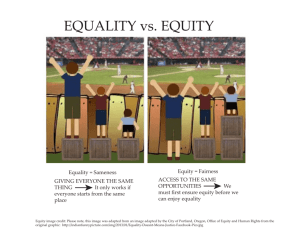
„International Research Project on Financing Quality in Health Care” InterQuality WP 2 Values/Benefits (development of methodology for cost and outcome of care measurement) „International Research Project on Financing Quality in Health Care” Joanna Lis Tomasz Hermanowski Warsaw Medical University WP2: Objectives • To solve methodological challenges raised by analysis of patient-level data in international comparative research • All Consortium partners will contribute with input on data availability and particular challenges in their countries • 9 months – from 3 to 12 months of the Project – 1 March 2011 – 31 Nov 2011 3 4 5 6 7 8 9 10 11 12 WP2: Objectives • Main objectives are to develop consensus and guidelines on: – common terminology and conceptual framework for costs and outcomes measurement and methods of measuring costs (task 2.1) – methods of evaluating economic efficiency, based on NICE guidelines for health technology evaluation and IQWIG efficiency frontier, including guidelines for measuring patient-relevant outcomes (PRO) (task 2.2) – methods of measuring quality and equity of healthcare (task 2.3) – methods of statistical analysis across countries, differentiating between country and policy effects (task 2.4) Task 2.1. common terminology and conceptual framework for costs and outcomes measurement and methods of measuring costs • Literature and methodology review, definition development: – – – – – Methods of outcomes& costs measurements Methods of evaluating economic efficiency Methods of measuring quality of healthcare Methods of measuring equity of healthcare Methods of statistical analyses/methods across countries, differentiating between country and policy effects Task 2.1. common terminology and conceptual framework for costs and outcomes measurement and methods of measuring costs • In order to avoid misunderstandings definitions of terms and conceptual framework of contemplated research will be agreed by all Consortium partners • The task will start with a review of basic reference sources, like ISPOR Book of Terms • (10 Man-Months) from 3 to 5 Months of the Project • Input from MUW (38Man-Month)s from 3 to 5 Month of the Project Task 2.2. methods of evaluating economic efficiency, measuring patientrelevant outcomes, costs, quality & equity in health care • Literature (and other available sources) review covering a range of fields: – Measuring costs – Economic evaluation (outcomes&costs) – Measuring quality – Measuring equity in areas of pharmaceutical care, hospital care, outpatient care and integrated care. Task 2.2. methods of evaluating economic efficiency, measuring patientrelevant outcomes, costs, quality & equity in health care • Measuring costs in healthcare systems – Methods of measuring costs will be described • Direct • Indirect – Availability of costs data will be analyzed by all Consortium Partners • Impact different healthcare systems organization on measuring costs Task 2.2. methods of evaluating economic efficiency, measuring patientrelevant outcomes, costs, quality & equity in health care • Economic evaluation (outcomes&costs) efficiency – Identification and description of efficency indicators – Methods of measuring patient-relevant outcomes will be reviewed (PRO) – Based on comparative analysis of existing methods of health technology evaluation guidelines for empirical analysis of cost efficiency will be developed Task 2.2. methods of evaluating economic efficiency, measuring patientrelevant outcomes, costs, quality & equity in healthcare • Measuring quality – Systematic review of publications will be performed • Scales and questionnaires – Generic » Health Profile » Utility Measurements – Specific – Results and databases, created in the framework of previous research projects, financed by FP 6 and 7, will be reviewed – Recommended methods for comparative analysis of healthcare quality will be proposed Task 2.2. methods of evaluating economic efficiency, measuring patientrelevant outcomes, costs, quality & equity in healthcare • Measuring equity: – Results of up-to-date research projects, financed by EU, OECD, WHO and World Bank by FP 6 and 7, will be completed – State-of-the art analytical methods will be used to evaluate equity of access to sectors of care and countries, up to now not analyzed Task 2.2. methods of evaluating economic efficiency, measuring patientrelevant outcomes, costs, quality & equity in healthcare • Partners will provide WP leader with results of review of literature perceived as relevant to country specific health care system issues • Input from collaborative partners (15Man-Month) from 3 to 5 Months of the Project: UI (2), MHH, UY, SDU, SPH, UniCT, CPME, EPF Input from collaborative partners 1. Consortium partners will be asked to execute, in the framework of collaborative work, systematic reviews of publications & other available sources for: – measuring of costs &outcomes, cost efficiency, quality and equity in healcare system (inpatient &oupatient care, integrated care and pharmaceutical care etc) – UI (2 Man-Months) at the end of two months of the project, WP1 Leader will be asked to provide protocol and preliminary results of literature review and catalogue of criteria related to costs, cost efficiency, quality and equity (deadline – end of 2 Project Months). 2. The objectives are: – Identify and execute a comprehensive review of all indicators / measures of quality used in healthcare systems worldwide and used in countries of collaborative partners – Compare impact of indicators / measures on the quality of healthcare system Task 2.2. Input from collaborative partners (15Man-Months) from 3 to 5 Month of the Project: Work package No Work package title Activity type Participant No Participant short name Person-month per participant 2 Start date of starting event 3 Values/Benefits (development of methodology for cost and outcome of care measurement) RTD 1 5 2 6 3 7 4 8 9 MUW UI MHH UY SDU SPH 54 2 2 2 2 3 UniCT CPME EPF 2 1 1 Task 2.2. Input from collaborative partners (15Man-Months) from 3 to 5 Month of the Project: Issues for discussion • Literature&other sources review: – Define period of review (1990-2010)? – Source od data: MEDLINE, EMBASE, Cochrane, Centre for Reviews and Dissemination database, HEED, DARE, AHRQ, OECD, WHO … but also • using literature references contained in clinical trial publications, • searching data published in specialist journals in the field of interests – Searching procedure(2 analysts, abstracts vs full versions, etc) • A common template for reporting the results of literature searches and databases Task 2.3. Comparative analysis of value/benefits measurement • A summary of the perceived strengths and weaknesses of methodology for cost and outcomes of care measurement will be developed • Areas of both consensus and disagreement will be identified • Country-specific considerations that make particular cost and outcomes of care measurement approaches desirable or particularly problematic will be identified • (10 Man-Months) from 6 to 8 Month of the Project Task 2.4. Methodology of statistical analysis across countries & Data Warehouse • On the basis of multilevel statistical analysis methodology, recommended methods for cross country comparative analysis will be identified • Adopted methods will enable Consortium Partners to identify country and policy effects in different data sets • In order to collect and analyze statistical data in uniform format, dedicated Data Warehouse will be prepared and used by all project participants • (18 Man-Months) from 9 to 12 Month of the Project WP2: Milestones • Data Warehouse test version start-up: – expected launch of data warehouse (test version) is after 10 Project Months • Methodology for Healthcare measurements development : – all guidelines and reports shall be validated and accepted by General Assembly in November 2011 (after 12 Project Months) WP2: Deliverables 1. Catalogue of definitions related to quality, equity, outcomes and costs evaluation as well as comparative value/benefit analysis (Guidelines on comparative evaluation) 2. Data Warehouse with Guidelines on statistical analysis across countries, differentiation between country and policy effects in different data sets, taking into consideration data availability and respecting confidentially requirements WP2: Deliverables • Hybrid database, managed by program CMS, which, in addition to reports of systematic reviews will put a bibliography of sources, with full description and possibly a short summary or review, links to other databases, descriptions of tools for measuring quality, efficiency, equality of access, etc. „International Research Project on Financing Quality in Health Care” joanna@fraktal.com.pl = 48 607 370 093 BACK-UPS Outcomes Natural measurements : E.g In oncology: OS, TTP, PFS … QALY (quality adjusted life year) HYE – (healthy year equivalent) SAVE – (saved young life equivalent) DALY - (disability-adjusted life years) … Costs Direct Medical Resources spent on medical services or products as a direct consequence of a disease or illness Resources directly consumed to produce a given outcome or consequence Direct Nonmedical Expenses related to the provision of medical care, but incurred outside the medical sector Transportation to a medical care facility Childcare Lodging • Indirect Costs: – Amounts spent or lost as an indirect consequence of illness or consumption of medical care • Lost wages due to sickness • Lost production • Premature death? • Intangible Costs – Pain and suffering – Social and emotional stress Utility QALY Health 1 Utility Therapy B Therapy A No treatment Death 0 Death with treatment A Death with no treatment time Death with treatment A Influences on quality of life (QoL) Disease Treatment Impairments (symptoms) Disability (functioning) HRQL Demographics Personality QoL Culture / economy Social Environment Stephen McKenna Galen Research, Manchester, UK Types of patient-reported outcomes Impairment (well-being) = Disability (functioning) Handicap (participation) Health-related quality of life (HRQL)/ (Health status) QoL HRQL ≠ QoL Rather than being HRQL or health status.. “QoL is a reflection of the way in which patients perceive and react to their health status and to other non-medical aspects of their lives.” Equity • Equity in health can defined as the absence of socially unjust or unfair health disparities. • However, because social justice and fairness can be interpreted differently by different people in different settings EQUITY in health can be defined as the absence of disparities in health (and in its key social determinants) that are systematically associated with social advantage/disadvantage. Health inequities systematically put populations who are already socially disadvantaged (for example, by virtue of being poor, female, or members of a disenfranchised racial, ethnic, or religious group) at further disadvantage with respect to their health.