Quantitative Analysis Lab Safety - Department of Chemistry and
advertisement

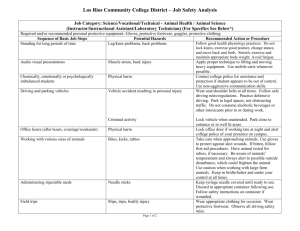
Laboratory Safety Revised August 2015 1 THE CHEMISTRY LABORATORY INCLUDES HAZARDS AND RISKS. Scientists understand the risks involved in the laboratory and have established a set of laboratory safety practices. This presentation summarizes some of the safety rules that scientists follow in the laboratory. 2 1. Personal Protective Equipment: What is required for you to wear when you work in the laboratory. 3 PPE – Personal Protective Equipment 4 Eye Protection • Contact lenses are OK if goggles or safety glasses are worn • Prescription glasses – you must wear goggles over them • Wish I'd Worn My Safety Glasses Video • Eye wash station Goggles: provide full Coverage and seal to your face Safety glasses must have side shields. 5 UV Goggles Cabinet – in most labs 6 Eye Protection, continued • Many labs in SSMB have student seating areas. Students must wear safety glasses when seated in these areas if any chemistry or cleanup is going on in the laboratory. You do not have to wear the glasses in the seating area at the start of lab during pre-lab lecture, provided there is no equipment or active experiments running. Once anyone starts an experiment everyone must wear safety glasses and lab coats until they exit the lab. This rule holds even when you are in the seating area working on results 7 Tie back long hair before entering the laboratory, don’t wear dangling jewelry. Yale physics student Michele Dufault was killed in a shop accident in April 2011 that would have been prevented had she tied her hair back 8 Foot Protection • Sandals, flip-flops, Crocs, open-toe and opentop (i.e. ballet flat) shoes and canvas shoes (i.e. Toms) are not appropriate. These will not adequately protect your feet. 9 Be Smart about the shoes you elect to wear to lab This person has on pants and closed toed-shoes but this would not be allowed in lab due to the exposed skin This person added socks, so this option covers your skin but only offers minimal protection This option looks better, but imagine chemicals being spilled into the top of these boots Your best options are sturdy leather footwear that covers the entire top of the foot or a sturdy running shoe. Result of Improper Footwear in a Laboratory Northwestern University, Evanston, IL July 2003 Your instructor will send you home to change if you do not have appropriate shoes or other required PPE. There should be no exposed skin on your feet. 11 Hand Protection: Chemically resistant Lab Gloves ✓ • Wear gloves of a material known to be resistant to permeation by the substances in use – nitrile is good for most of our laboratory classes. Latex gloves are not allowed. • Inspect each glove for small holes or tears before use. • When you spill on your glove or tear it, change it immediately. Throw gloves away any time you take them off. 12 Karen Wetterhahn (October 16, 1948 – June 8, 1997) Dartmouth College The latex gloves she was wearing were not resistant to methyl mercury – it passed through the glove, through her skin, entered her blood system and resulted in her death. 13 Use of Gloves Remove gloves before handling objects such as doorknobs, telephones, pens, computer keyboards, pH meter or other electronic buttons, or phones while in lab. It might be convenient to have one gloved hand and one ungloved hand to do procedures where these kinds of things are used. • Throw away gloves anytime you take them off. • You should expect to use several pairs of gloves in any given lab period. 14 Wash your hands! • Always, even after wearing gloves, wash your hands with soap and water before leaving the lab. 15 Lab Coats and Long Pants • All students in a Chemistry lab must be wearing long pants that cover your entire leg. • All students must wear a kneelength lab coat. These are available in the bookstores. • No skin should be showing from your waist down. • Keep your lab coat in a plastic zip lock bag to prevent contamination of items in your book bag 16 UCLA Lab Fire: December 29, 2008 Sheri Sangji was using this plastic syringe to transfer tert-butyllithium. This was not the correct procedure, because this compound is well-known to ignite if it is comes in contact with air. The syringe plunger dropped out of the syringe and the reagent ignited. Sheri died January 16, 2009 of severe burns. She was wearing nitrile gloves but no lab coat. The students assisting her did not remember to put her under the safety shower. 17 Lessons from UCLA accident Lessons: know the proper procedures for transferring dangerous reagents. Wear your lab coat and safety glasses at all times in the lab. Know where safety shower and other emergency equipment is – you may need to be the one who is ready to act when your lab mate is unable to help themselves. 18 2. Eyewash and Safety Shower: Know where these are in your lab. 19 Eyewash / Safety Shower The eyewash is on the left. Pull the handle and a fountain of water will appear that you can use to bathe your eyes. The safety shower is on the right. Pull the handle and water will start spraying from the shower head on the ceiling. There’s no drain in the floor – we only do this in emergencies, because a flood of water will have to be cleaned up. 20 Eye Wash 21 Safety Shower 22 3. Chemical Fume Hoods: You must do your experiment in the hood if any of your reagents are flammable, have harmful fumes or present a splash or explosion hazard. 23 Using the Fume Hoods properly This window/bar is called the sash. If this is not saying NORMAL, then the hood is not protecting you. Keeping the sash and sliding panels in proper position keeps this NORMAL, otherwise the alarm goes off. If the alarm goes off, you need to reposition things to the correct positions, then press the “mute” button to reset the controller. The sash should never be raised above the green “operation” level when you 24 are working in the hood. In use, side-to-side panel used as shield Closed, not in use ✓ In use, sash (window) raised to less than 18 inches ✓ ✓ Don’t open side shields to make one big window. × 25 Fume Hood Use • Video on use of Fume Hood 26 • When using a laboratory hood, set the equipment and chemicals back at least 6 inches. • Never lean in and/or put your head in the hood when you are working. This is worse than doing the experiment with no hood at all. • Keep liquid reagent containers in trays to catch all spills and drips 27 4. Know the risks of the chemical reagents you are working with 28 Labels are important Older NFPA labels with hazard levels identified on the diamond New GHS label system with pictogram hazards Even if it seems obvious. In the chemistry lab, nothing is ever obvious. Students may want to bring a sharpie to lab for writing labels on solutions that they make. At a minimum, a student made solution should have the chemical name, the date, and the student’s initials. 29 Older: NFPA Diamond (National Fire Protection Agency) 30 New Standard: GHS (Globally Harmonized System) • Under GHS regulations, each chemical product label must contain the following: Product Identifier, Signal Word, Pictogram, Hazard Statement, and Supplier Information. 31 GHS and NFPA Numbering Systems • It is very important that you realize that the number codes for NFPA and GHS HazCom are not the same. • For the NFPA diamond 4 is the most hazardous, for GHS HazCom 1 is most hazardous. • Since many chemicals have a long shelf life, you may find many containers with the NFPA diamond in the years ahead. The GHS HazCom labeling began in 2014. 32 MSDS (SDS) • Provides procedures for handling or working with that substance in a safe manner • Includes physical data melting point, boiling point, flash point, etc. toxicity, health effects, first aid reactivity, storage, disposal protective equipment, & spill-handling procedures. • Many MSDS sheets can be easily found with a google search. • SDS has replaced the MSDS acronym 33 5. Fire Safety 34 Fire Alarms – know the location of one close to your lab 35 Fire Extinguishers – we have several in the labs and in the hallways. 36 37 Types of Fire Extinguishers This is a special fire extinguisher for combustible metal fires. It is a type D fire extinguisher. You won’t need to use this unless you work in a research lab with combustible metals. Most of our fire extinguishers are ABC. It contains a dry powder to put out the kinds of fires we might encounter in the chemistry labs where we have class. 38 Demonstration of PASS and Use of a Fire Extinguisher https://www.youtube.com/watch?v=ZCSms-jyOao 39 Student Reaction in a Fire Although we want you to be informed on the operation of a fire extinguisher, we do not expect you to use it. If a fire is ignited in your area, the proper STUDENT response is to: 1) Notify everyone in the room 2) Proceed to the nearest exit and pull the nearest fire alarm 3) Evacuate the building 4) Assemble in front of the library or in the YWCA parking lot 40 Working with flames • Never leave experiments unattended unless you take special precautions to avoid accidents and you notify the responsible individuals. • Flames are never allowed when flammable gases or liquids are in use. • Always alert others before lighting a flame. • Never leave a flame unattended under any circumstances. 41 6. Gas Cylinder Safety 42 Gas Cylinders • http://www.youtube.com/watch?v=mReuQCuJNQQ • A gas cylinder will become a missile if the valve is broken or cracked. • Gas cylinders must always be securely chained to a wall. The chain should not be loose. • If a cylinder is not in use or is going to be moved, it must be capped to protect you and everyone else in the building. • Do not attempt to move a gas cylinder or to adjust regulators attached to a cylinder. • Do not open a gas valve if it does not have a regulator attached to the valve • Gas cylinders in general chemistry and organic labs are likely part of the operation of instruments; students should not attempt to adjust the regulators or move the cylinders. Exposed valve regulator 43 Gas Cylinder Safety × ✓ 44 7. Disposal Procedures 45 Broken Glassware • Always check your glassware and discard any with chips, breaks, or obvious flaws. • Throw away broken glassware into special glass waste containers 46 Waste Disposal • Waste containers are provided for chemical waste generated in laboratories • Some things can go down the sink, some can’t. Always check with your instructor. • Care must be used to avoid mixing incompatible chemicals such as – Acids with Bases – Oxidizers and Flammables – Water reactive and aqueous solutions – Cyanides and acids 47 University of Maryland September 26, 2011 • Students were conducting an experiment with nitric acid and sulfuric acid was added into a chemical waste container, causing a violent chemical reaction sparked a small fire in and near the laboratory chemical ventilation hood. • Two female students were injured as a result • Sustained first- and second-degree chemical burns and superficial cuts. 48 8. How to be a good lab citizen 49 Must-have habits for good lab students • Begin with a clean work surface with your instructions clearly posted and available; have a clear, clean work space and eliminate unnecessary books, book bags, equipment, etc. • Return all lab materials and equipment to their proper places after use as instructed; clean your lab space as instructed by your teacher or lab instructor/supervisor leaving it in proper order for the next person. 50 Keep your lab area clean. × Throw away used paper towels and used gloves, immediately. × Don’t block the floor in front of the eyewash/shower station. × Don’t leave cords dangling because someone will trip over them. × Don’t leave things in the floor because someone will trip over it. 51 Don’t put anything on your face or in your mouth while you’re in lab. • Take care not to ingest anything in the laboratory! • Food, gum, beverages, candy, and tobacco products are never allowed in the laboratory. • Don’t apply makeup, chapstick, lotion, or anything to your face or hands during lab. Wash your hands with soap then leave the lab before touching your face or other exposed skin. • In the seating areas of labs, sealed water bottles or beverages should be in and stay in a backpack for the duration of the lab. 52 Stay aware of what’s happening around you while you’re working in the lab. • Don’t use any distracting electronic devices while in laboratory. If you touch your phone during lab, you’re contaminating it with whatever chemicals you’ve been working with. • Do not wear earbuds in the lab. You need to be able to hear important announcements, especially in an emergency or when a safety concern is addressed. 53 Chemical Spills • Notify your instructor and your neighbors if you spill chemicals on the floor or bench. • Don’t try to clean it up yourself. Your instructor may need to use a specially designed chemical spill kit. • Each lab is equipped with a spill kit that contains absorbent materials such as spill mats, snakes, and vermiculite. 54 Texas Tech January 7, 2010 • Conducting research funded by the U.S. Department of Homeland Security on energetic / explosive compounds • Attempting to produce 100 times more of an explosive compound than the informal lab limit (100mg) Lesson: Follow instructions in the lab. 55 Students must report any injuries, big or small. • Report all injuries to the instructor. We will not call emergency services unless the instructor determines it is a serious injury. • An incident report will be filled out whether it is small or serious. 56 Injury procedure, continued • First Aid kits are available in the lab with band aids and other items for treating small cuts and burns. • If it is a serious injury, your instructor will call campus emergency services, 843-953-5611. Our campus officers will work with the instructor and/or injured student to determine whether or not 911 EMS should be called in. 57 Once again, the number to call in an emergency is: 843-953-5611 Please take a moment now to program this number into your cell phones 58 • The number one responsibility of your lab instructor is to monitor maintain a safe working environment for everyone in the lab • Your lab instructor will be in attendance during your lab walking around to monitor PPE and experiments. Everyone must wear the required PPE at all times. There are no exceptions, so don’t ask for one. • Do not ask permission to attend lab without PPE. It is your responsibility to be properly attired and if you are not, you will be asked to leave. • If your instructor asks you to cease a behavior or activity that they deem unsafe and you do not comply, your instructor will tell you to leave the lab. 59 Report any concerns • If you have any safety concerns about the lab you are working in or the people working around you, you can contact: – Your lab instructor – Dr. Brooke Van Horn– Head of the departmental safety committee – Dr. Pamela Riggs-Gelasco – Department Chair for Chemistry and Biochemistry – Dr. Jim Deavor, Associate Dean of the School of Science and Mathematics. 60 Summary • Know & follow the Safety Rules • Look for & recognize safety hazards • Take proactive steps to minimize the risk of injury from the hazards. 61 Chemical storage • • • • Flammables/combustibles Acids Bases Oxidizers 62 Mineral Acids – inorganic acids Mineral Acids can be stored together except for Nitric Acid, which must be stored by itself because it is also strong oxidizer. Strong Mineral Acids • Hydrochloric Acid • Hydrobromic Acid • Hydroiodic Acid • Nitric Acid • Perchloric Acid • Sulfuric Acid Weak Mineral Acids • Phosphoric Acid • Boric Acid • Hydrofluoric Acid Organic Acids • Organic Acids can be stored together, but they must be stored separately from both mineral acids and nitric acid. - e.g., Formic acid, Acetic acid, Lactic acid, Citric acid, Oxalic acid, etc. Storing Acids and Bases • Mineral Acids can be stored in the same cabinet as Bases, as long as they are physically isolated from each other. • If your lab contains some of each of these categories of acids, you should have the following separate cabinets: 1. Mineral Acids + Bases 2. Organic Acids 3. Nitric Acid (Strong Oxidizer) The color of the bottle cap has a meaning • • • • Cap color corresponds to concentrated solution of: Red: Nitric Acid Blue: Hydrochloric Acid Yellow: Sulfuric Acid Brown: Acetic Acid : Phosphoric Acid Also one base: • Green: Ammonium Hydroxide Acid bottles (+ ammonium hydroxide) • If you prepare an aqueous solution and store it in a glass or plastic bottle, you shouldn’t use one of these cap colors. Instead, try to use clear, black, orange, or purple caps. Nitric Acid • If nitric acid is mixed with a flammable organic compound, such as acetic acid, the heat from the oxidation and neutralization reactions is enough to ignite the flammable material. • Nitric acid also slowly destroys its red plastic bottle cap. Always replace with a new red cap. • Nitric acid may turn yellow over time because of the release of nitrogen dioxide on exposure to light. The yellow color does not affect the product’s usefulness in the school laboratory. Sulfuric Acid • Concentrated sulfuric acid is a strong dehydrating agent. Because of its strong ability to remove water, it reacts violently with many organic materials such as sugar, wood, and paper. • If sulfuric acid has turned brown, it has probably been contaminated with an organic material and its purity should be in question. University of Maryland September 26, 2011 • Students were conducting an experiment with nitric acid and sulfuric acid was added into a chemical waste container, causing a violent chemical reaction sparked a small fire in and near the laboratory chemical ventilation hood. • Two students were injured as a result; they sustained first- and second-degree chemical burns and superficial cuts. Hydrochloric Acid • Concentrated hydrochloric acid fumes continuously and cannot be stored without releasing hydrochloric acid vapor. These fumes are responsible for most of the corrosion damage in your chemical storeroom. Storing hydrochloric acid in a wood or plastic-lined acid cabinet is a must. Hydrochloric acid fumes will quickly corrode metal cabinets. • Hydrochloric acid fumes mixed with ammonia fumes will react to form ammonium chloride clouds and possibly toxic chloramines. Open containers of these two reagents should not be in the same hood. Report any concerns • If you have any safety concerns about the lab you are working in or the people working around you, you can contact: – Your lab instructor – Dr. Neal Tonks– Head of the departmental safety committee – Dr. Pamela Riggs-Gelasco – Department Chair for Chemistry and Biochemistry – Dr. Jim Deavor, Associate Dean of the School of Science and Mathematics. 72