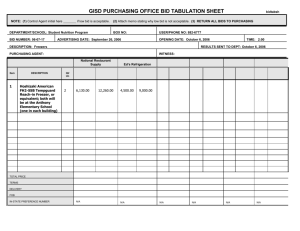
KX2-391

A Phase I trial of KX2-391, a novel non-ATP competitive substrate-pocket directed SRC inhibitor, in patients with advanced malignancies
A. A. Adjei, R. B. Cohen, R. Kurzrock, G. S. Gordon, D. Hangauer,
L. Dyster, G. Fetterly, S. Barrientes, D. S. Hong, A. Naing
Roswell Park Cancer Inst, Buffalo, NY; Fox Chase Cancer Center,
Philadelphia, PA; MD Anderson Cancer Center, Houston, TX;
Kinex Pharmaceuticals, Buffalo, NY
FOX CHASE
Cancer Center 1
Disclosure Information
ASCO ANNUAL MEETING 2009
Alex A. Adjei
I have no financial relationships to disclose.
Src signaling in tumor cells
Yeatman, Nat Rev Cancer, 2004
Src Kinase in Cancer
• There are over 500 serine and tyrosine kinases in humans; Src is a member of the tyrosine kinase family and the 1 st oncogene discovered
• Src activity is high in a majority of primary tumors with further increases during metastasis
• Since Src activity generally increases as cells progress from benign to invasive to metastatic, Src inhibition may inhibit primary tumor growth as well as metastasis
4
Src kinase Inhibitors in the clinic
• Dasatinib (Sprycel): (approved for resistant CML,
Phase I-II in solid tumors)
• Bosutinib (SKI-606): (Phase III in resistant CML,
Phase I-II in solid tumors)
• AZD0530: (Phase I-II in solid tumors)
• XL228: (Phase 1 in resistant CML & solid tumors)
5
KX2-391
Mechanism of action
Non-ATP competitive Src signaling inhibitor
2 nd MOA (not a kinase) currently under investigation
6
Efficacy of KX2-391 in Solid Tumor Cell Lines
Human Tumor Cell Line KX2-391 GI50 (nM)
HT29 (Colon)
SKOV-3 (Ovarian)
25
10
PC3-MM2 (Prostate) 9
L3.6pl (Pancreas)
MDA-MB-231 (Breast)
A549 (Lung)
HuH7 (liver)
769-P (kidney)
25
20
9
9
45
KX2-391 has very potent activity (<50 nM) against a broad range of solid tumor cell lines
KX2-391 Compared to Dasatinib in
Resistant Solid Tumor Cell Lines
Human Tumor Cell Line
H460 (NSCLC))
H226 (NSCLC)
HCT116 (colon)
SW620 (colon)
KX2-391 IC50--IC90 (nM)
51--162
98--490
31--195
109--903
Average IC50 = 72 nM
Dasatinib IC50--IC90 (nM)
90--48,880
163--34,340
880--not reached
2,418--2,940
Average IC50 = 888 nM
Literature reported Dasatinib resistant cell lines:
1) Johnson et al, Clin. Cancer Res 2005; 11(19), 6924
2) Serrels et al, Mol Cancer Ther 2006; 5(12), 3014
Dasatinib 10X less potent than KX2-391 at IC50 and ca. 100X less potent at IC90
KX2-391 Compared to Dasatinib in Leukemia and Multiple Myeloma Cell lines
Human Liquid Tumor Cell
Line
K562 (CML)
K562R (Gleevec resistant
CML)
Ba/F3 + T315I
(Gleevec & Dasatinib
Resistant CML)
CCRF-HSB-2 (ALL)
KG-1 (AML)
KX2-391 GI50 (nM)
13
0.64
35
Dasatinib GI50 (nM)
0.37
0.81
Inactive
12
16
Inactive
Inactive
RPMI8226 (Multiple
Myeloma)
40 Inactive
KX2-391 has a second MOA beyond Src signaling inhibition that might provide broader activity than dasatinib
KX2-391 Pre-clinical Toxicology
Oral continuous bid dosing 28-day toxicity studies
Rodent
GI Toxicity is Dose-Limiting
No cardiotoxicity
No renal toxicity
Non-Rodent (Dog)
GI and Hematologic Toxicities are Dose-Limiting
No cardiotoxicity
No renal toxicity
10
Objectives of Phase I Trial
Primary Objective:
• To define the maximum tolerated dose (MTD) of KX2-391 in patients with advanced refractory malignancies
Secondary Objectives:
• To determine the safety and tolerability of KX2-391 given as single and multiple doses as an oral solution in patients with advanced refractory malignancies
• To characterize the pharmacokinetic profile after single and multiple oral dosing of KX2-391
• To determine the biological effects of KX2-391
• To evaluate antitumor activity of KX2-391
11
Trial Design
• Multi-center Phase 1 trial
• Standard ‘3+3’ dose escalation design
• Dosing
• Single oral dose, followed by one week evaluation
• Then BID oral dosing for 3 of 4 weeks
• Efficacy evaluation every 2 cycles (8 weeks)
• PK evaluation after single and multiple doses
12
Inclusion/Exclusion Criteria
• Adults over age 18 years of age
• Confirmed advanced solid tumor or lymphoma for which standard curative or palliative measure do not exist or are no longer effective
• ECOG performance status of 0-2
• Adequate bone marrow reserve
• Adequate liver and renal function
• No investigational agents within 28 days of first day of study
• No recent hormone therapy
• Not using moderate or strong P450 modulators (inducers or inhibitors) within 2 weeks or 5 half-lives (whichever is shorter)
• No major surgery within 4 weeks
• No major GI disease
• No signs or symptoms of other major diseases or toxicities
13
Patient Demographics
Characteristics
Age Median (range)
Sex
Median # of previous chemotherapy regimens
Median # of previous XRT regimens
Most common cancers
Colo-rectal
Head and Neck
Pancreas
Breast
NSCLC
Thyroid
Other
Results
60 (32-80) years
15 males/ 22 females
3
2
Number of patients
4
3
3
3
4
4
16
14
Dose Group
2 mg BID
5 mg BID
10 mg BID
20 mg BID
40 mg BID
80 mg BID
60 mg BID
50 mg BID
TOTAL
Dose Escalation
# patients
4
4
7
3
4
4
37
5
6
# with DLTs
0
0
0
0
0
2
6
2
2
# with SD> 4 cycles
1
1
1
1
1 MTD
2
3
1
11
15
Pharmacokinetics of KX2-391
Conclusions: KX2-391 PK is dose-dependent and linear with increasing dose.
Terminal T1/2 is approximately 4 hr. T max
– 1 hour
Treatment Related Adverse Events
Adverse Event
AST increased
ALT increased
Anorexia
Fatigue
Lymphopenia
Nausea
Anemia
Leukopenia
Alk phos increased
Pancytopenia
Febrile Neutropenia
Dizziness
Flatulence
Neutropenia
Grade 1/2*
7
4
3
4
2
5
4
2
3
1
2
2
1
Grade 3/4*
3
3
1
2
2
1
1
Total
10
7
3
2
2
2
2
4
4
3
5
4
4
3
1
Other Grade 3 or 4 events in one patient: Abdominal pain, bilirubin increased, edema, failure to thrive, hyponatremia, mucositis, rash, thrombocytopenia
17
* Number of patients
Dose Limiting Toxicities
• Dose Limiting Toxicities at 50, 60 and 80 mg BID
• Grade 4 Neutropenia – 2 patients (60 mg and 80 mg dose)
• Grade 3 elevated ALT/AST – 2 patients (50 and 60 mg dose)
• Grade 4 thrombocytopenia – 1 patient (50 mg dose)
• Grade 3 ALT/AST/Failure to Thrive – 1 patient (80 mg dose)
• Serious Adverse Events (possibly related, at any dose level)
• Febrile Neutropenia, pancytopenia, hyponatremia – 1 patient
• Febrile Neutropenia, pancytopenia, edema – 1 patient
• Neutropenia and mucositis – 1 patient
• Rash – 1 patient
• Failure to thrive – 1 patient
• All DLTs and SAEs reversible within 7 days
• No pulmonary edema or cardiotoxicity noted
18
Efficacy Results (N = 37)
• Treatment duration
• Median 2 cycles (range 1 – 11)
• 11 pts treated for 4 or more cycles
• 6 pts treated for 6 or more cycles
• Patients with suggestion of efficacy
• Prostate – PSA decline : 205 to 39, 6 cycles
• Pancreas – CA19-9 decline : 38,838 to 267, 4 cycles
• Ovary – CA-125 decline : 665 to 207, 4+ cycles
• Thyroid – 3 patients on for 8, 8, and 6+ cycles
• Appendiceal carcinoid – 11+ cycles
• Melanoma – 6 cycles
• Merkel cell – mixed response at 2 cycles
19
Prostate Cancer Patient
Change in PSA
250
200
150
100
450
400
350
300
50
0
On Trial Off Trial
7/
3/
20
07
8/
3/
20
07
9/
3/
20
07
10
/3
/2
00
7
11
/3
/2
00
7
12
/3
/2
00
7
1/
3/
20
08
2/
3/
20
08
3/
3/
20
08
4/
3/
20
08
5/
3/
20
08
6/
3/
20
08
7/
3/
20
08
20
Pancreas Cancer Patient
Change in CA19-9
On Trial Off Trial
21
Ovary Cancer Patient
Change in CA125
On Trial
22
Conclusions
• KX2-391 is well-tolerated at a dose of 40 mg po BID for 3 out of 4 weeks
• Pulmonary and cardiac toxicities have not been seen
• Linear and dose-dependent PK profile
• Half-life of 4 hours supports BID dosing
• Demonstrates preliminary evidence of antitumor activity
• Should be further evaluated in Phase II trials
• Future trials planned
• Prostate
• Pancreas
• Breast
• AML
23
Acknowledgements
• To the patients and their families
• To the staff:
• MDAnderson
Saneese K Stephen PA , MPAS , MPA
Chandtip Chandhasin, PhD
Senait N Fessahaye , Data Coordinator
• Roswell Park Cancer Institute
Grace Dy, MD
Wen Wee Ma, MD
Kathy Galus, Pharm D
Deborah Yoon, BSN
• Kinex Pharmaceuticals
Allen Barnett, PhD
Kristen Thomas, MS
Johnson Lau, MD, PhD
Jane Fang, MD
• Fox Chase Cancer Center
Ranee Mehra, MD
Holly Dushkin, MD
Igor Astsaturov, MD, PhD
Elizabeth Zeidler, RN
Christine Malizzia
• AHRM, Inc
Laura Dalfonso
Ben Finkel
Mary Caparole
Amy Hayward
24