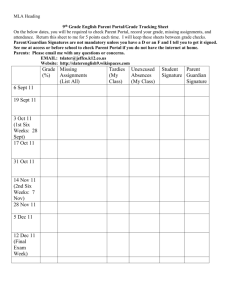
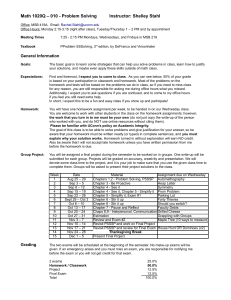
CHEMISTRY 115C BASIC CHEMISTRY CONCEPTS
advertisement

CHEMISTRY 121 INTRODUCTION TO CHEMISTRY Fall 2014 Evening Session: Lecture -- Tues (6-9)/Thurs (6-7), Lab Thurs. (7-9, KUL 105)) I. General Information: Instructor: Dana Beatty Prerequisite: Math 99 Office: KUL 108 (classroom) Office hours: Tues & Thurs 5:15-6:00 or by appointment E-mail: dbeatty@whatcom.ctc.edu – best way to contact me Phone extension: 3883 Website: www.chemistry121.pbworks.com II. Required Materials: Textbook: General, Organic, and Biological Chemistry, 4th ed, Timberlake (2013) Scientific Calculator -- A calculator that can perform basic functions such as scientific (exponential) notation and logarithms and the ability to properly use it (e.g. Texas Instruments TI30XA or a Graphing calculator Safety goggles – must be ANZI approved (sold at bookstore) Bound Composition Book – to be used as your lab notebook for lab work III. Course Description: This is an introductory chemistry course for those who have either not had a previous chemistry course or need to refresh their understanding of chemistry. One misconception about introductory courses is that they are easy. While this may be the case for some courses, it is not the case in this one. It is important that you consistently attend lecture and lab, and that you actively participate in the problemsolving portions of the class. At times, you will be required to work in small groups on learning activities. Laboratory work will allow students to learn techniques of analysis that they can use to explore the concepts learned in the classroom IV. Course Goals: The goals of this course are: 1. To establish the basic principles associated with modern chemistry and to begin laying the foundations of general inorganic chemistry. 2. To prepare students for further chemistry courses (Chem 161, 131) 3. To provide students interested in pursuing a career in the health sciences the background knowledge necessary for further course work. V. Objectives: As a result of this course, you should be able to Solve mathematical problems in chemistry using scientific measuring units and rules for calculations. Sort matter into categories and differentiate between matter and energy Describe basic atomic structure and how it relates to the structure of the periodic table Describe the basic types of bonds holding substances together, write names and formulas for compounds based on the type of bonding, and explain the relationship between the type of bonding present in a substance and its physical properties Write and interpret chemical equations for chemical reactions Calculate quantities of reactants and products involved in chemical reactions Describe the properties and behavior of gases and use gas law equations to solve problems Describe the nature and properties of solutions (especially aqueous solutions), and perform calculations relating to solution concentration 1 Describe how the rates of chemical reactions can be changed and how chemical equilibria can be shifted Describe the properties of acids and bases and the concept of pH Gain competency in basic laboratory skills VI. Course Requirements Attendance: Lecture meets for 3 hours Tuesdays and 1 hour Thursdays (before the lab). Lecture is extremely important, and attendance will be taken. Materials that you need to bring to every lecture session include your text and calculator as, at times, you will break into small groups to work on problems form the text. Students missing more than 2 laboratory periods will receive a failing grade in the class. Lecture notes: We will be covering chapters 1-3 and 5-10. Students may obtain lecture notes, exam study guides, additional practice problems and other materials from the course website. It is advised that you print off the lecture notes for the chapter before coming to class. Homework: Students are responsible for reading the assigned chapters and completing the assigned in‐ chapter exercises and end‐of‐chapter problems to prepare for quizzes and exams. Homework is not submitted or graded; however, it is expected that students complete the homework assignments in order to develop the necessary problem‐solving skills to successfully complete the course. Quizzes: In order for me to assess your understanding of the course content covered in lecture and text assignments, a series of 15 point quizzes will be given. Some of these may not be pre-announced. Sharing calculators is not allowed during quizzes and using devices that function beyond performing mathematical calculations or are capable of a wireless connection, examples include cell phones and PDA’s, is prohibited. The lowest quiz score of the quarter will be dropped when calculating your grade. Exams: Four in-class exams will be given. The exam score that I record for these exams will be the percentage of points earned. Again, sharing calculators or using cell phones as calculator is not allowed. On exams, any equations needed to answer exam questions will be provided. Any material covered earlier in the course is assumed to be common knowledge, and you can expect to see it again both in lectures and exams. o What if I miss an exam? You can only make-up one missed exam in the testing center. Make‐up exams will only be allowed for legitimate reasons -- excused absences (e.g. illness with a doctor’s note), a death in the family -- as determined by the instructor. You are expected to take the exam on your own time at the testing center, and must do so immediately as I can arrange. You will be eligible to take a make-up exam only if: 1. You directly contact me sometime before the beginning of the exam you will miss. 2. You provide a note with a detailed explanation of why you missed the exam when you attend the next class. Laboratory Work: Check the CHEM 121 Lab Schedule for the experiment to be carried out each week. Laboratory sessions are scheduled for 2 hours (7-9 on Thursdays) and you should plan to be in lab for the full 2 hours. Anyone arriving to lab later than 15 minutes will NOT be allowed to perform the scheduled experiment. You are required to go through a safety orientation during the first laboratory session the first week of class. You will not be allowed to perform any experiments if you have not gone through this orientation. If you miss the lab orientation, it is your responsibility to contact me for alternative arrangements. Individuals not adhering to lab safety procedures will be asked to leave the lab. Procedures for each lab are found in the Chemistry 121 Instructional Packet, which may be purchased at the bookstore, or on the website 2 VII. Students may only attend their scheduled lab period. No make‐up labs are allowed! Students missing more than 2 experiments will receive a failing grade in the course. Laboratory notebook - You must keep a bound laboratory notebook (see the lab information handout for format). At the end of each lab session, you must show me your notebook so that I can sign-off on the work that you have done. Your lab notebook is due on the day of the final exam and is worth 25 points. Students must come to lab prepared, having read the entire experiment and prepared the lab notebook. To ensure that you have read the lab procedures before each experiment, a prelaboratory quiz worth 4 points will be given, you may use your pre-lab write-up for this quiz. If your pre-lab write-up is not completed, you will not be allowed to perform the experiment. After each experiment, all calculations must be completed and post‐lab questions must be answered. Completed lab reports are due by the start of lab the following Thursday. Lab reports submitted during the lab will be penalized 10%; other late lab reports will be penalized 10% per day. Lab reports must be turned in directly to me. Do NOT use any student drop-box. Grading/Evaluation: The course grade will be determined based on total points earned from the following: POINT BREAKDOWN (subject to change) 4 exams at 100 points each: 400 points quizzes at 15 points each: 75 points minimum (5 quizzes minimum) 9 lab reports at 15 points each: 225 points 1 lab notebook: 25 points Using the following grading scale: A AB+ B B- 94% and up 90% to 93% 87% to 89% 84% to 86% 80% to 83% C+ C CD+ D D- 77% to 79% 74% to 76% 70% to 73% 67% to 69% 64% to 66% 60% to 63% If your percentage falls between grades, such as 93.6% or 79.4%, other factors that will be used to determine your grade include (but are not limited to) attendance, class participation and lab reports turned in on time. Students taking the course on an S/U basis are expected to earn at least a "C-" to receive a "Satisfactory" grade. If at any time during the quarter you would like to have your grade calculated, please do not hesitate to ask me. Other : An incomplete grade (I) is available for a number of verifiable personal emergencies so long as the student has shown sufficient effort and satisfactory progress as defined by the instructor. Satisfactory progress includes, but is not limited to; assignments up-to-date, 70% average on exams, constructive class participation, etc. An incomplete grade will not be considered an option if fewer than 2/3 of the lab experiments have been completed and if the average grade for those completed labs is less than 85%. If circumstances in your life prevent you from succeeding in this course at this time, you should withdraw and try the course at another time. If you stop attending class and do not officially withdraw you will receive an F The last day to drop the class without receiving a “W” on your transcript is April 24, 2013 3 VIII. General Policies Cheating: Cheating involves submitting work that you fraudulently claim as your own. If you are caught cheating on a quiz or test, you will receive a score of zero and your name will be forwarded to the VicePresident of Educational Services. While I encourage the discussion of course material with other classmates as an aid in learning the material, answers generated by these discussions must be written in your own words. If you submit a lab report where you and others have identically worded (or near identically worded/paraphrased) work, then points will be deducted. If this continues on future assignments, you will receive 0 your name will be forwarded to the Vice-President of Educational Services. Cell Phone Policy: All cell phones must be powered off and kept out of sight during the lecture and lab (note that placing such a device on your lap does not qualify as being out of sight). If there is a need for your cell phone to be on (an example would be you are on-call, or your child is at home with a sitter) inform me before class. Americans with Disabilities Act (ADA): Any student with a documented disability requiring class accommodations should contact the Disability Support Services office in the Entry and Advising Center (LDC 116) at 360-383-3080 Disclaimer: Course content may vary from this outline to meet the class’ needs. EXAM & QUIZ SCHEDULE (subject to change) DATE TOPIC COVERED POSSIBLE POINTS Exam 1 Oct 7 Chapters 1 ,2,3 100 Points Exam 2 Oct 28 Chapters 5 & 6 100 Points Exam 3 Nov. 18 Chapters 7 & 8 100 Points Exam 4 Dec. 4 Chapters 9 & 10 100 Points LECTURE SCHEDULE (subject to change) CHAPTER DATES COVERED Chapter 1 Sept. 16 -23 Chapter 2 Sept 23-25 Chapter 3 Sept 30 – Oct 2 Chapter 5 Oct 7 - 14 Chapter 6 Oct. 16 -23 Chapter 7 Oct 28 – Nov 4 Chapter 8 Nov 4 - 13 Chapter 9 Nov 18 –25 Chapter 10 Nov 25 – Dec 2 4 LABORATORY SCHEDULE (subject to change) All lab reports are due by the beginning of class. Any lab report turned in later in the day or the next school day loses 2 points. If a lab report is 2 school days late, it loses 4 points; if 3 school days late, it loses 8 points; and if 4 school days late, it loses 16 points. Lab reports turned in after 4 school days will receive no points, but I will note that you put forth the effort to complete that lab report. DATE Sept. 18 Sept 25 Oct. 2 Oct. 9 Oct. 16 Oct 23 Oct 30 Nov. 6 Nov. 13 Nov. 20 Nov. 27 EXPERIMENT Lab Orientation Laboratory Measurements Heating Water Investigation of the Elements Decomposition and Formation of Compounds Synthesis of Magnesium Oxide Molecular Weight of a Volatile Liquid Conductance Equilibrium/Le Chatelier’s Principle pH of Solutions Thanksgiving, no lab DUE DATE NA Oct.2 Oct. 9 Oct. 16 Oct 23 Oct 30 Nov. 6 Nov.13 Nov 20 Dec 2 TEXTBOOK ASSIGNMENTS Chapter 1 2 3 5 6 7 8 9 10 Assigned Problems 1.13, 1.15, 1.17, 1.25, 1.29, 1.31, 1.33, 1.39, 1.41, 1.45, 1.46, 1.51c, 1.53, 1.57, 1.61, 1.65a, 1.79, 1.85, 1.87, 1.91 2.1,2.5, 2.9, 2.13, 2.15, 2.17, 2.21, 2.24, 2.29, 2.31, 2.33a, 2.35b, 2.36c, 2.37d, 2.40a, 2.41 a,d, 2.46, 2.53, 2.55, 2.57, 2.79a, 2.83 3.5, 3.9, 3.12, 3.16, 3.17, 3.20, 3.21, 3.22, 3.27, 3.29, 3.31-33, 3.41, 3.43, 3.51, 3.53, 3.57, 3.59, 3.69, 3.71, 3.75, 3.76, 3.83, 3.101, 3.103, 3.107, 3.117 5.1-3, 5.9, 5.13, 5.17, 5.19, 5.21, 5.25, 5.27, 5.29, 5.35, 5.37, 5.40, 5.41, 5.43, 5.45, 5.51, 5.53, 5.59, 5.61, 5.63, 5.65, 5.69, 5.72, 5.73, 5.75, 5,77, 5.79, 5.85, 5.87, 5.89, 5.91,b,c, 5.93, 5.107, 5.109, 5.121, 5.130, 5.131 6.1, 6.3, 6.5, 6.7, 6.11, 6.13, 6.17, 6.21, 6.23, 6.25, 6.31a,e, 6.33a,c, 6.37, 6.39c,d, 6.41b, 6.47, 6.51, 6.53, 6.57, 6.59, 6.67, 6.71a,b, 6.73, 6.75, 6.77, 6.79, 6.86, 6.89, 6.92, 6.101, 6.111, 6.115, 6.117,c, 6.123, 6.128, 7.1-9, 7.12-14, 7.15a,c, 7.17a,c, 7.21, 7.23, 7.25a,b, 7.27a,b, 7.31, 7.35, 7.37, 7.45, 7.47, 7.49, 7.51, 7.53, 7.55, 7.59, 7.63, 7.67, 7.69, 7.72, 7.77, 7.83, 7.84, 7.89, 7.93, 7.102, 7.103, 7.105 8.1-3, 8.5-9, 8.11, 8.13, 8.21-23, 8.27, 8.29, 8.31-33, 8.35, 8.37, 8.39a, 8.41, 8.49, 8.51, 8.53, 8.55a,b, 8.56a,b, 8.57a,c, 8.61, 8.65-67, 8.69, 8.77, 8.78, 8.80-84, 8.88, 8.91, 8.93, 8.97, 8.99b, 8.100a, 8.105a,c 9.7-10, 9.5, 9.17, 9.19, 9.21, 9.23, 9.27, 9.29, 9.31, 9.33-9.36, 9.49, 9.55, 9.57 10.1-14, 10.17-20, 10.23, 10.25, 10.27, 10.29-33, 10.35, 10.37, 10.39, 10.41, 10.43-45, 10.49, 10.51, 10.53, 10.55, 10.59, 10.71, 10.75-76, 10.85, 10.89, 10.103 5