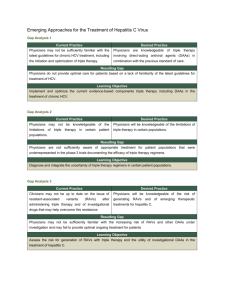
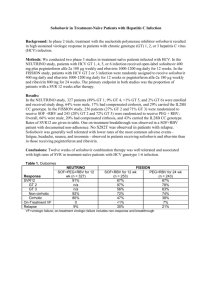
C_YURDAYDIN
advertisement

Treating a patient with liver cirrhosis “What is happening in real life ?” Mr. UB, 35 yrs old Turkish engineer living in Switzerland, highly intelligent, typical patient of the “informative age” we live in; knows “a lot” on hepatitis C treatment, is managed in Switzerland and Turkey by Swiss and Turkish physicians Ankara Uni. 16 March 2005: • ALT: 84 (N< 40), AST: 64 (N <40) • Alk. Phosphatase: 193 (N <120) • GGT: 115 (N <50) • HBsAg and Ab negative, anti HCV positive • T. Bilirubin: 11.2 (N <17) • Prothrombin activity: 54%, INR: 1.3 • Albumin: 4g/dL • Ultrasound: consistent with cirrhosis, diffuse splenomegaly, no ascites • Liver biopsy (Metavir): F4, A1 • Endoscopy: Grade II esophageal varices • Child- Pugh score: 6 • HCV RNA: 4 480 000 IU/mL (7.65 log) • Viral genotype: 1a Ankara Uni. What would you do ? No treatment, FU with US for HCC and preparation for future liver tx 2. Tx with standard dose and duration of pegylated INF/riba 3. Tx with standard dose and prolonged duration of pegylated INF/riba 4. Titrated tx with peg/riba 5. Start with triple tx 1. Ankara Uni. Patient asked for “maximal” therapy ! 15.04.2005: Treatment started with PEG2a 180µg/qw + Ribavirin 1200 mg/qd with the intention to use higher doses of ribavirin, subject to patient’s tolerability Ankara Uni. Tx start date: 15.04.2005 01.04.05 17.05.05 10.06.05 08.07.05 Hb 17.2 14.8 14.1 13.5 Plat. 71 51 44 52 WBC 6.9 3.8 3.1 2.4 Neut. 4.7 1.4 1.2 1.0 T. Bili 30.8 22.0 22.6 24.8 ALT 116 124 107 119 INR 1.3 1.4 1.4 1.2 HCV RNA 7.65 log NA NA 3.11 log At month 3: HCV RNA ↓ by > 2 log Ursodeoxycholic acid (1000mg/qd) added in 08/2005, Ribavirin dose increased to 1600 mg/day Ankara Uni. •Undetectable since at least week 20 of treatment commencement •After 48 weeks of treatment HCV undetectable Ankara Uni. What would you do now ? 1. Discontinue treatment 2. Continue tx with the same regimen to week 72 3. Continue tx with “maintenace dose” for Pegasys and Ribavirin dose to 90 µg Ankara Uni. Situation in depth discussed with patient. Patient did not want to risk relapse. It was decided to continue treatment. Patient did not want to decrease dose of neither Pegasys nor Ribavirin. Ankara Uni. Tx start date: 15.04.2005 April 2005 July 2005 Sep 2005 March 2006 June 2006 ALT 116 119 67 43 38 AST 77 110 88 98 69 T. Bili 30.8 24.8 27.7 34.4 38.1 INR 1.3 1.2 1.3 1.5 1.6 Albumin 4.0 NA NA 3.3 2.7 HCV RNA 7.65 log 3.11 log < 15 IU/mL < 15 IU/mL < 15 IU/mL Ankara Uni. Patient Case 1 • HPI: A 58-year-old woman with newly diagnosed HCV. She had a blood transfusion at age 28. Current symptoms: fatigue and myalgias • PMH: mild depression • Medications: – Escitalopram 10 mg po QD, atorvastatin 10mg po QD • Evaluation: – – – – – HCV genotype 1b HCV RNA: 1.6 million IU/mL CBC/platelets and TSH: normal Ultrasound: increased echogenicity in liver, otherwise normal Liver biopsy: bridging fibrosis • Patient decides to start therapy – Telaprevir + PEG-IFN + RBV 1 0 What would you tell this patient about her chances of SVR with a course of PI-based therapy? A. B. C. D. SVR rate cannot be estimated SVR rate ~25%-35% SVR rate ~40%-50% SVR rate ~70% HCV Treatment Decisions for Protease Inhibitors Pros Cons • PIs substantially increase chance of SVR across a majority of patient groups • Suboptimal response rates or limited/no data in several populations • PIs shorten duration of therapy in many • Successful treatment improves morbidity and mortality – HCV-HIV co-infection, tranplant, decompensated cirrhotics • Complicated regimens, challenging AEs, and DDIs • Risk of resistance if therapy fails: impact on future options? Identifying Candidates For Triple Therapy Host factors Treatment regimen Age, gender, race obesity, co-morbidities Genetic factors (IL28B and ITPA) PEG-IFN Ribavirin DAA Factors to Consider In Treatment Decisions Disease features Fibrosis, steatosis, co-infection (HBV, HIV) Viral factors Genotype / Subtype Quasispecies / Resistance Viral load Candidates for PI-Based Triple Therapy • Chronic HCV genotype 1 • Fulfill criteria for PEG-IFN/RBV therapy • If cirrhotic, should be well-compensated – No variceal hemorrhage, ascites, encephalopathy • Ability to adhere to treatment goals and monitoring • Safety and efficacy has not been established in HIV or HBV coinfected, pediatric, or pregnant patients or in organ transplant recipients Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. SPRINT 2: SVR and Relapse Rates (ITT) Boceprevir: Treatment-naïve HCV G1 patients SVR* Relapse Rate P < 0.001 P = 0.004 P < 0.001 P = 0.04 125 311 37 162 211 316 21/232 213 311 18/230 Non-Black Patients *All Pts who received treatment Data from Poordad F, et al. N Engl J Med. 2011;364(13):1195-1206. 12 52 2/14 22 52 3/25 Black Patients 29 55 6 35 ADVANCE: Higher SVR Rates in Patients Achieving eRVR Telaprevir: Treatment-naïve HCV G1 patients 97 100 P<0.001 90 SVR, % 80 89 75 T12PR PR48 31% 70 60 50 54 44 39 40 30 10 0 271/363 158/361 Treatment-naïve Overall 189/212 28/29 eRVR+ 82/151 130/332 eRVR- • 58% of Pts eligible for RGT (received 24 weeks of TVR-based regimen) • SVR rates 89% in T12PR Data from Jacobson IM, et al. N Engl J Med. 2011;364:2405-2416. Prior to starting PI-based therapy, how would you counsel the patient regarding management of her depression? A. Patients on antidepressants cannot receive PI-based therapy B. St. John’s wort is okay to use with PIs C. Some antidepressants may need to have doses adjusted during treatment with a PI D. Patients with a history of depression should not receive PI-based therapy Pre-Treatment Evaluation: DDI with PIs • BOC and TVR are CYP3A4 inhibitors • Drug interactions may affect blood levels of either PI or co-administered drug Inhibito 10 Drug + Inhibitor Inhibitor blocks the function of the CYP enzyme P450 r AUC 5 Inducer AUC 1 1 • Caution is needed with ALL co-administered medications – Review package inserts for interaction lists – Reconcile patient medication list – Patient needs to communicate new meds started by other health care providers – Other resource: www.hep-druginteractions.org Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Ghany MG, et al. Hepatology. 2011;54(4):1433-1444. Figure adapted from: Back D. Drug-drug interactions (in relation to HCV). Presented at: 7th International Workshop on HIV & Hepatitis Co-infection; June 1-3, 2011; Milan, Italy. Lecture. Patient Case 1 (continued) • Medications: escitalopram 10 mg po QD, atorvastatin 10mg po QD – Do you need to make any changes to the patient’s medications? Drug Interactions Considerations Concomitant Drug Class Drug(s) with Interaction PI Involved CONTRAINDICATED: •May lead to loss of virologic response ( concentrations of PIs) St. John’s Wort Antidepressants Trazodone, Desipramine Effect on Concentration of PI or Concomitant Drug BOC or TVR trazodone, desipramine: • Dizziness, hypotension, and syncope • Use with caution; consider lower doses of trazodone/desipramine Escitalopram TVR ↔ telaprevir, escitalopram: •Dose of escitalopram may need to be adjusted Lovastatin, Simvastatin BOC or TVR CONTRAINDICATED: • Potential for myopathy including rhabdomyolysis BOC atorvastatin: •Titrate slowly; max dose of 20 mg/d TVR CONTRAINDICATED Statins Atorvastatin Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Birth Control and Pregnancy During Triple Therapy • Systemic hormonal contraceptives should not be relied on as an effective method of contraception – 2 alternative methods of contraception (barrier methods or IUDs) should be used during treatment and for 6 months after • Triple therapy is contraindicated in pregnant women and men whose female partners are pregnant – Ribavirin may cause birth defects and fetal death – Negative pregnancy test prior to therapy & monthly during therapy Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Telaprevir: Treatment-Naïve & Prior Relapse Patients Chronic HCV Genotype 1, telaprevir 750 mg (two 375 mg tablets) orally 3 times daily (7-9 hrs apart) with food (~ 20 gm fat†) 0 Treatment Decision Points Initiate antiviral treatment 4 End of 4 Weeks 12 End of 12 Weeks Telaprevir + PEG-IFN + RBV 4-week HCV-RNA 24 End of 24 Weeks PEG-IFN + RBV 12-week HCV-RNA eRVR undetectable at weeks 4 & 12 Responseguided therapy 48 End of 48 Weeks PEG-IFN + RBV Treatment complete @ 24 weeks Tx-naïve w/ cirrhosis‡ †Ingest food within 30 minutes prior to dose ~20 gm fat: Bagel w/cream cheese; 1/2 cup nuts; 3 tbsp peanut butter; 1 cup ice cream; 2 oz American or cheddar cheese; 2 oz potato chips; 1/2 cup trail mix. Treat for 48 weeks ‡Treatment-naïve patients with cirrhosis who have undetectable HCV-RNA at weeks 4 and 12 may benefit from an additional 36 weeks of PEG-IFN/RBV (48 weeks total) For telaprevir, HCV-RNA at wk 4 and wk 12 determine duration of therapy Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Telaprevir: Treatment-Naïve & Prior Relapse Patients Chronic HCV Genotype 1, telaprevir 750 mg (two 375 mg tablets) orally 3 times daily (7-9 hrs apart) with food (~ 20 gm fat) 0 Treatment Decision Points Initiate antiviral treatment 4 End of 4 Weeks 12 End of 12 Weeks Telaprevir + PEG-IFN + RBV 4-week HCV-RNA 24 End of 24 Weeks PEG-IFN + RBV 48 End of 48 Weeks PEG-IFN + RBV 12-week HCV-RNA Detectable Responseguided therapy ≤1000 IU/mL at weeks 4 and/or 12 Treatment complete @ 48 weeks For telaprevir, HCV-RNA at wk 4 and wk 12 determine duration of therapy Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Telaprevir: Stopping Rules 0 Treatment Decision Points Initiate antiviral treatment Stopping Rules 4 End of 4 Weeks 12 End of 12 Weeks Telaprevir + PEG-IFN + RBV 24 End of 24 Weeks PEG-IFN + RBV 4-week HCV-RNA 12-week HCV-RNA 24-week HCV-RNA > 1000 IU/mL > 1000 IU/mL Detectable Treatment failure Treatment failure Treatment failure Stop Stop Stop Apply to all patients Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. 48 End of 48 Weeks Boceprevir: Treatment-Naïve Patients Chronic HCV Genotype 1, boceprevir 800 mg (four 200-mg capsules) 3 times daily (7-9 hrs apart) with food 0 4 Treatment Decision Points Initiate antiviral treatment 8 12 End of 8 Weeks PEGIFN+RBV End of 12 Weeks 24 28 36 48 End of 24 Weeks Boceprevir + PEG-IFN + RBV 8-week HCV-RNA Responseguided therapy 24-week HCV-RNA Continue Treatment Undetectable Undetectable Treatment complete @ 28 wks For boceprevir, HCV-RNA at wk 8 and wk 24 determine duration of therapy Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Boceprevir: Treatment-Naïve Patients Chronic HCV Genotype 1, boceprevir 800 mg (four 200 mg capsules) 3 times daily (7-9 hrs apart) with food 0 4 Treatment Decision Points Initiate antiviral treatment 8 End of 8 Weeks PEGIFN+RBV 12 End of 12 Weeks 24 28 End of 24 Weeks 36 48 End of 48 Wks PEGIFN+ RBV Boceprevir + PEG-IFN + RBV 24-week HCV-RNA 8-week HCV-RNA Stop BOC at Wk 36 Responseguided therapy Continue Treatment Detectable Undetectable Treatment complete @ 48 wks For boceprevir, HCV-RNA at wk 8 and wk 24 determine duration of therapy Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Boceprevir: Treatment-Naïve Patients Chronic HCV Genotype 1, boceprevir 800 mg (four 200 mg capsules) 3 times daily (7-9 hrs apart) with food 0 4 Treatment Decision Points Initiate antiviral treatment 8 End of 8 Weeks PEGIFN+RBV Poorly IFNresponsive* 12 End of 12 Weeks 24 28 End of 24 Weeks 36 48 End of 48 Wks Boceprevir + PEG-IFN + RBV Triple therapy for 44 weeks < 1 log10 IU/mL decline in viral load at Wk 4 Treatment complete @ 48 wks *Standard stopping rules assessed at Wk 12 and 24 still apply Assess interferon responsiveness after lead-in with PEG-IFN/RBV Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Boceprevir: Stopping Rules 4 0 Treatment Decision Points Initiate antiviral treatment Stopping Rules Apply to all patients 8 End of 8 Weeks PEGIFN +RBV 12 End of 12 Weeks 24 End of 24 Weeks 28 36 End of 48 Wks ±BOC + PEG-IFN+ RBV Boceprevir + PEG-IFN + RBV 12-week HCV-RNA 24-week HCV-RNA ≥ 100 IU/mL Detectable Treatment failure Treatment failure Stop Stop Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. 48 HCV-RNA Levels and Lab Assays • “Undetectable” result is required for assessing RGT eligibility • Below LLOQ but still “detectable” is not sufficient to shorten therapy—ie, patient should continue for full 48 wks LLOQ Values for Various Assays Assay Name LLOQ Roche COBAS® AmpliPrep/COBAS® TaqMan® HCV Test 43 IU/mL Roche COBAS® TaqMan® 25 IU/mL† HCV Test, v2.0 Abbott RealTime HCV Assay 12 IU/mL † Usually considered 25 IU/mL, but 23 IU/mL per FDA-approved label COBAS® AmpliPrep/COBAS® TaqMan® HCV Test. Roche Molecular Diagnostics. http://molecular.roche.com / assays/Pages/COBASAmpliPrepCOBASTaqManHCVTest.aspx. Accessed July 19, 2011. Harrington P, Naeger L. Frequency and Clinical Relevance of Detectable/<LLOQ HCV RNA in Boceprevir and Telaprevir Trials. United States Food and Drug Administration (FDA), FDA Division of Antiviral Products; June 30, 2011. Telaprevir and Boceprevir Adverse Events Adverse Events Reported More Frequently vs PEG-IFN/RBV Telaprevir1 Adverse Event, % Telaprevir-Containing Arms (n = 1797) PEG-IFN/RBV Arm (n = 493) Rash 56 34 Pruritus 47 28 Anemia* 36 17 Anorectal AEs** 29 7 *No EPO used in TVR trials; EPO commonly used in BOC trials **hemorrhoids, anorectal discomfort, anal pruritus, and rectal burning Boceprevir2 Adverse Event, % Boceprevir-Containing Arms (n = 734) PEG-IFN/RBV Arm (n = 363) Anemia* 45-50 20-30 Dysgeusia 35-44 11-16 1Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. 2Victrelis Telaprevir: Rash Summary • In most subjects, the rash was mild-moderate – Typically eczematous, maculopapular, and papular-lichenoid • 4% severe—resulted in discontinuation of telaprevir in 6% of subjects • < 1% SJS or drug rash with eosinophilia and systemic symptoms (DRESS) • Can occur at anytime • Improvement occurs after dosing completion or D/C; may take weeks for complete resolution Advisory Committee Briefing Document for NDA 201-917 Telaprevir 375 mg tablets. Silver Spring, MD; April 1, 2011. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugs AdvisoryCommittee/UCM252561.pdf. Accessed April 26, 2011. Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Drug Rash Due to Telaprevir Slide courtesy of Dr. Stuart Gordon Rash Management Plan: Telaprevir Rash Description Management Mild to moderate rashes — — — • Continue all drugs; TVR dose should not be reduced or interrupted Monitor for rash progression or development of systemic symptoms Oral antihistamines and/or topical corticosteroids Systemic corticosteroids are not recommended* Severe rash — Discontinue TVR, continue PEG-IFN/RBV — If no improvement within 7 days (or earlier if indicated), consider D/C of PEG-IFN and/or RBV — Oral antihistamines and/or topical corticosteroids • Systemic corticosteroids are not recommended* — Consider dermatology consult Serious skin reactions (SJS or DRESS): Discontinue all medications immediately; Refer for urgent medical care All patients with rash Consider good skin care practices: limit sun exposure, wear loosefitting clothing, use oatmeal or baking soda baths, apply moisturizers at least twice daily after bathing, laundry with mild, unscented detergents *Systemic corticosteroids & telaprevir drug-drug interactions: prednisone/methylprednisolone (CYP3A substrates) and telaprevir (potent CYP3A inhibitor) – plasma concentrations of corticosteroids can be increased significantly. Systemic dexamethasone (induces CYP3A) can decrease telaprevir plasma concentrations (may result in loss of therapeutic effect) Vertex Medical Information Letter: Rash in patients receiving Incivek (telaprevir) combination treatment. Published July 2011. Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. On-Treatment Consideration for Managing Triple Therapy in the Treatment-Experienced Patient Patient Case 2 • HPI: 58-year-old woman with HCV Genotype 1a – Biopsy in 2003 showed cirrhosis – No evidence of clinical decompensation • MELD = 8 and platelet count was 115,000/mm3 • CT scan with contrast reveals nodular liver but no HCC – Her most recent treatment course was PEG-IFN alfa-2a + RBV • She is anxious to start therapy with an HCV protease inhibitor Considerations for TreatmentExperienced Patients in 2011-2012 • Likelihood of response to PI/PEG-IFN/RBV – Previous response pattern – Viral factors • HCV genotype and HCV RNA level – Host factors • IL28B genotype • Race • Obesity/insulin resistance • Likelihood of clinical disease progression – Advanced fibrosis / cirrhosis • Likelihood of tolerating PEG-IFN/RBV + protease inhibitor Definitions of Prior Response Response Definition Evaluated in clinical trials? Partial Response HCV RNA decline ≥ 2 log10 IU/mL from baseline at week 12, but never achieved undetectable HCV RNA BOC: Yes TVR: Yes Relapse HCV RNA undetectable at the end of therapy, but detectable HCV RNA during follow-up BOC: Yes TVR: Yes Null Response HCV RNA decline < 2 log10 IU/mL from baseline at week 12 of prior therapy BOC: Yes* TVR: Yes *PROVIDE study, AASLD 2011 Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co., Inc.; 2011. Advisory Committee Briefing Document for NDA 201-917 Telaprevir 375 mg tablets. Silver Spring, MD; April 1, 2011. http://www.fda.gov /downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252561 .pdf. Accessed April 26, 2011. VierlingJM, et al. Poster presented at: AASLD The Liver Meeting 2011; November 4-8, 2011; San Francisco, CA. Poster 931. Patient Case 2 (continued) • Prior treatment course resulted in ~ 2.1 log10 reduction in HCV RNA after 12 weeks with no further decrease after 24 weeks; stopped at 24 weeks – She developed hypothyroidism and is on replacement therapy – During prior therapy, Hgb decline was ~4 g/dL, but RBV was not reduced – No PEG-IFN dose reductions – IL28B genotype is not known RESPOND-2: SVR in Prior Relapsers and Prior Partial Responders Prior Relapsers n/N= Prior Partial Responders 15 51 72 105 77 103 2 29 27 57 30 58 PR48 BOC RGT BOC/ PR48 PR48 BOC RGT BOC/ PR48 HCV G1 patients with previous treatment failure Bacon B, et al. N Engl J Med. 2011;364(13):1207-1217. Copyright © 2011 Massachusetts Medical Society. REALIZE: SVR in Prior Relapsers, Prior Partial Responders, and Prior Null Responders Prior Relapsers * Prior Partial Responders Prior Null Responders * SVR (%) * * * n/N = * Pbo/ T12/ LI T12/ PR48 PR48 PR48 Pbo/ PR48 T12/ LI T12/ PR48 PR48 Pbo/ PR48 T12/ LI T12/ PR48 PR48 16/68 121/145 124/141 4/27 29/49 2/37 21/72 Data from Zeuzem S, et al. N Engl J Med. 2011;364(25):2417-2428. 26/48 25/75 *P < 0.001 vs Pbo/PR48 REALIZE: SVR by Baseline Fibrosis Stage and Prior Response Prior Partial Responders Prior Null Responders SVR (%) Prior Relapsers n/N = Stage 12/38 144/167 2/15 53/62 2/15 48/57 3/17 34/47 0/5 10/18 1/5 11/32 1/18 24/59 0/9 15/38 1/10 No, minimal Bridging Cirrhosis No, minimal Bridging Cirrhosis No, minimal Bridging or portal fibrosis or portal fibrosis or portal fibrosis fibrosis fibrosis fibrosis 7/50 Cirrhosis Zeuzem S, et al. Presented at: EASL: The International Liver Congress 2011; March 30-April 3, 2011; Berlin, Germany. Oral Presentation 5. Patient Case 2 (continued) • She is a prior “partial responder” with slightly more than 2 log10 decline – Last treatment ~ 2003 – Biopsy showed cirrhosis – In the interval, she has developed type 2 diabetes mellitus, controlled with metformin – She is also taking simvastatin and lisinopril • Current Hgb 13.8 g/dL, platelet count 111,000/mm3 and HCV RNA 1.74 million IU/mL (6.24 log10) • Is there a role for IL28B testing prior to treatment? SVR Rates by IL28B Genotype in Subjects who Previously Failed Therapy Genetic Variant Near the Gene Encoding Interferon-lambda-3 (IL28Brs12979860, a C to T Change) IL28B Genotype RESPOND-2: Boceprevir PR48 BOC-RGT BOC-PR48 C/C 46 (6/13) 79 (22/28) 77(17/22) C/T 17 (5/29) 61 (38/62) 73 (48/66) T/T 50 (5/10) 55 (6/11) 72 (13/18) IL28B Genotype REALIZE: Telaprevir SVR, % (n/N) SVR, % (n/N) PR48 TVR12-PR48 (pooled regimens) C/C 29 (5/17) 79 (60/76) C/T 16 (9/58) 60 (160/266) T/T 13 (4/30) 61 (49/80) Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Patient Case 2 (continued) • She initiates PEG-IFN + RBV 600 mg po BID with plans to add boceprevir 800 mg po every 8 hours after treatment week 4 – Simvastatin is held due to concerns for possible drug-drug interaction with boceprevir • After completion of the “lead-in” phase, her Hgb level is 10.5 g/dL and her HCV RNA is 89,000 IU/mL (4.95 log10) Baseline 6.24 log10 Week 4 4.95 log10 Difference 1.29 log10 decrease • What does this lead-in response mean? Interferon Responsiveness Was Predictive of SVR With Boceprevir SVR, % From RESPOND-2: Patients who failed previous therapy with PEG-IFN/RBV Week 4 Response Responsiveness ≥ 1 log10 Decline in VL IFN Response < 1 log10 Decline in VL Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Telaprevir: SVR by Prior Response Category and Week-4 Response to PEG-IFN/RBV Lead-in <1 log10 HCV RNA Reduction at Week 4 100 ≥1 log10 HCV RNA Reduction at Week 4 94 Prior relapsers Prior partial responders Prior null responders Patients (%) 80 62 60 59 56 54 40 20 15 0 SVR rate SVR rate REALIZE Study Foster GR, et al. Presented at: EASL: The International Liver Congress 2011; March 30 - April 3, 2011; Berlin, Germany. Oral Presentation. 6. Patient Case 2 (continued) • Boceprevir is added to her regimen at treatment week 5 • After 8 weeks of treatment, she reports fatigue, dyspnea on exertion, and shortness of breath • Hgb level is now 8.9 g/dL For this patient, what is the most appropriate initial management of her anemia? A. B. C. D. E. Stop the PI Decrease the dose of RBV Decrease the dose of the PI Epoetin alfa 40,000 IU SC weekly B and D Boceprevir: Anemia Summary • Higher rates of anemia in patients treated with boceprevir Adverse Event, % Anemia BOC-Containing Arms (n = 734) PEG-IFN/RBV Arm (n = 363) 45-50 20-30 • Patients treated with boceprevir had: – Average additional decrease of Hgb of approximately 1 g/dL – Higher frequency of hemoglobin reductions to Grade 3 or higher toxicity • Mechanism of anemia thought to be result of bone marrow suppressive effect associated with boceprevir, not due to RBC hemolysis, as observed with ribavirin • Management strategies during clinical trials: – RBV dose reduction or erythropoietin alone or in combination – RBC transfusion US Food and Drug Administration; April 27, 2011. http://www.fda.gov/downloads/AdvisoryCommittees/Committees MeetingMaterials/Drugs/AntiviralDrugsAdvisoryCommittee/UCM252341.pdf. Accessed April 28, 2011. Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Boceprevir: SVR According to EPO Use and RBV Dose Reduction Retrospective analysis of SPRINT-2 and RESPOND-2 Previously Untreated (SPRINT-2) BOC arms only Previous Treatment-Failures (RESPOND-2) BOC arms only 100 100 74 60 71 58 40 20 0 212 363 80 68 SVR (%) SVR (%) 80 78 95 129 29 37 109 153 30 44 No EPO Both Neither R dose anemia alone reduction alone Anemia 60 80 83 47 59 5 6 72 73 48 67 19 26 50 40 20 0 83 165 No EPO Both Neither R dose anemia alone reduction alone Anemia N = 1097 treatment-naïve; N = 403 previous-treatment-failure Sulkowski MS, et al. Poster presented at: EASL: The International Liver Congress 2011; March 30-April 3, 2011; Berlin, Germany. Poster 1800. Telaprevir: Anemia Summary • Higher rates of anemia in patients treated with telaprevir Adverse Event, % Anemia TVR-Containing Arms (n = 1797) PEG-IFN/RBV Arm (n = 493) 36 17 • Patients treated with telaprevir had: – A higher frequency of hemoglobin reductions to Grade 3 or higher (55% vs 25%) – A higher frequency of Hgb level < 8.5 g/dL (14% vs 5%) – More anemia-related SAEs (2.5% vs < 1%) – A higher frequency of anemia-related discontinuations (4% vs < 1%) • EPO was not used during clinical trials • Anemia was managed with RBV dose reduction Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Advisory Committee Briefing Document for NDA 201-917 Telaprevir 375 mg tablets.Silver Spring, MD; April 1, 2011. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AntiviralDrugs AdvisoryCommittee/UCM252561.pdf. Accessed April 26, 2011. Anemia Management Recommendations With PI-based Therapy • Monitor closely for Hgb < 10 g/dL • CBC pretreatment, every 2 weeks until treatment week 8, then monthly • Primary strategy: RBV dose reduction – If RBV is D/C, BOC or TVR also must be D/C – Do not reduce PI dose to manage anemia • Hgb < 8.5 g/dL: discontinue all therapy • Once RBV dose reduction has been tried, EPO can be considered (off-label) PEGASYS (peginterferon alfa-2a) injection for subcutaneous use [package insert]. South San Francisco, CA: Genentech, Inc.; September 2011. PegIntron (peginterferon alfa-2b) injection, powder for solution for subcutaneous use [package insert]. Whitehouse Station, NJ: Schering Corporation; 2011. Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Patient Case 2 (continued) • Her anemia was managed with RBV dose reduction from 600 mg PO BID to 800 mg/day – After 2 weeks, her Hgb was stable but symptoms continued – Epoetin alfa 40,000 IU SC weekly was added • After 12 weeks of treatment, her HCV RNA is detectable at 38 IU/mL – Should treatment continue? – If yes, how long should she be treated with boceprevir and/or with PEG-IFN/RBV? Triple Therapy Should Be Stopped in Patients With Insufficient Viral Response Telaprevir* Timepoint Criteria for Stopping Action Week 4 or 12 HCV-RNA > 1000 IU/mL Discontinue TVR/PEG-IFN/RBV Week 24 HCV-RNA detectable Discontinue PEG-IFN/RBV Boceprevir** Timepoint Criteria for Stopping Action Week 12 HCV-RNA ≥ 100 IU/mL Discontinue BOC/PEG-IFN/RBV Week 24 Confirmed, detectable HCV-RNA Discontinue BOC/PEG-IFN/RBV * Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. ** Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Emergence of Pre-Existing Resistant Variants During Treatment With DAA HCV RNA Baseline HCV RNA Start Treatment Viral Breakthrough X XXX X X X X X Before Treatment Time on Treatment With DAA Alone Resistant virus Sensitive virus Adapted from: Forum for Collaborative HIV Research and Hepatitis C Virus Drug Development Advisory Group. A New Perspective on HCV Drug Resistance: Multiple Paths to Sustained Viriologic Response: Resistance Can Be Overcome [PowerPoint]. Washington, DC; 2011. SVR Is the Best Way to Prevent Resistance • Never use PIs as monotherapy; always use PIs in combination with PEG-IFN/RBV • Maximize adherence to all 3 drugs in regimen – Multidisciplinary approach to management: physicians, NPs, PAs, nurses, and pharmacists • Aggressive management of side effects • Careful assessment of viral response and application of “stopping rules” • Resistance is typically observed in persons for whom PEG-IFN/RBV is not effective – Novel treatment paradigms will be needed Boceprevir: Previous Partial Responders or Relapsers Chronic HCV Genotype 1, boceprevir 800 mg (four 200 mg capsules) 3 times daily (7-9 hrs apart) with food 4 Treatment Decision Points Initiate antiviral treatment 8 End of 8 Weeks PEGIFN+RBV 12 End of 12 Weeks 24 28 36 48 End of 24 Weeks Boceprevir + PEG-IFN + RBV 24-week HCV-RNA 8-week HCV-RNA Responseguided therapy Continue Treatment Undetectable Undetectable Treatment complete @ 36 wks For boceprevir, HCV-RNA at wk 8 and wk 24 determine duration of therapy Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Boceprevir: Previous Partial Responders or Relapsers Chronic HCV Genotype 1, boceprevir 800 mg (four 200 mg capsules) 3 times daily (7-9 hrs apart) with food 0 4 Treatment Decision Points Initiate antiviral treatment 8 End of 8 Weeks PEGIFN+RBV 12 End of 12 Weeks 24 28 End of 24 Weeks 36 48 End of 48 Wks PEGIFN+ RBV Boceprevir + PEG-IFN + RBV 24-week HCV-RNA 8-week HCV-RNA Stop BOC at Wk 36 Responseguided therapy Continue Treatment Detectable Undetectable Treatment complete @ 48 wks For boceprevir, HCV-RNA at wk 8 and wk 24 determine duration of therapy Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Boceprevir: Nonresponders & Cirrhotics Chronic HCV Genotype 1, boceprevir 800 mg (four 200 mg capsules) 3 times daily (7-9 hrs apart) with food 0 Treatment Decision Points Initiate antiviral treatment 4 8 End of 8 Weeks PEGIFN+RBV 12 End of 12 Weeks 24 28 End of 24 Weeks 36 48 End of 48 Wks Boceprevir + PEG-IFN + RBV Treatment complete @ 48 wks Prior null responders Triple therapy for 44 weeks Patients with compensated cirrhosis Treatment complete @ 48 wks RGT was not studied in patients with < 2 log10 HCV-RNA decline by wk 12 during prior therapy with PEG-IFN/RBV Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck & Co, Inc.; 2011. Telaprevir: Treatment of Prior Partial & Null Responders Chronic HCV Genotype 1, telaprevir 750 mg (two 375 mg tablets) orally 3 times daily (7-9 hrs apart) with food (~ 20 gm fat) 0 Treatment Decision Points Initiate antiviral treatment 4 End of 4 Weeks 12 End of 12 Weeks Telaprevir + PEG-IFN + RBV 24 End of 24 Weeks 48 End of 48 Weeks PEG-IFN + RBV Triple therapy for 12 weeks No RGT in partial and null responder patients with TVR Incivek (telaprevir) film coated tablets [package insert]. Cambridge, MA: Vertex Pharmaceuticals; 2011. Treatment complete @ 48 weeks Patient Case 2 (continued) • After 24 weeks of treatment, she has improved symptoms with minimal shortness of breath and no major complaints – Hgb 11.2 g/dL – Epoetin alfa is decreased to 20,000 IU weekly – HCV RNA not detected • She will continue triple therapy with boceprevir + PEG-IFN/RBV for a total of 48 weeks Treatment-Experienced Patients: Take-Home Points • Higher SVR rates with boceprevir or telaprevir + PEG-IFN/RBV • Response rate is highly dependent on prior IFN/RBV response and fibrosis stage: – Relapser: 70%-88% – Partial responder: 40%-59% – Null responder: 29%-33% • Potential for increased side effects • Potential for resistance associated variants • For patients that fail PI, combination DAAs may be an option in the future Victrelis (boceprevir) capsules [package insert]. Whitehouse Station, NJ: Merck and Co, Inc.; 2011. Zeuzem S, et al. N Engl J Med. 2011;364(25):2417-2428.