Graph theory as a method of improving chemistry and mathematics
advertisement

Graph theory as a method of
improving chemistry and
mathematics curricula
Franka M. Brückler,
Dept. of Mathematics, University of
Zagreb (Croatia)
Vladimir Stilinović,
Dept. of Chemistry, University of
Zagreb (Croatia)
Problem(s)
• school mathematics: dull? too
complicated? to technical?
• various subjects taught in school:
to separated from each other?
from the real life?
• possible solutions?
Fun in school
• fun and math/chemistry - a
contradiction?
• can you draw the picture traversing each
line only once? – Eulerian tours
• is it possible to traverse a chessboard with
a knight so that each field is visited once?
– Hamiltonian circuits
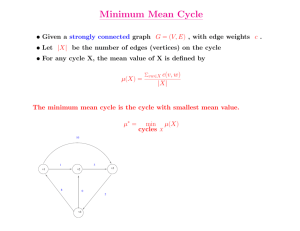
Graphs
• vertices (set V) and edges (set E) – drawn
as points and lines
• the set of edges in an (undirected) graph
can be considered as a subset of P(V)
consisting of one- and two-member sets
• history: Euler, Cayley
Basic notions
• adjacency – u,v adjacent if {u,v} edge
• vertex degrees – number of adjacent vertices
• paths – sequences u1u2...un such that each
{ui,ui+1} is and edge + no multiple edges
• circuits – closed paths
• cycles – circuits with all vertices appearing only
once
• simple graphs – no loops and no multiple edges
• connected graphs – every two vertices
connected by a path
• trees – connected graph without cycles
Graphs in chemistry
• molecular (structural) graphs (often: hydrogensupressed)
• degree of a vertex = valence of atom
• reaction graphs – union of the molecular graphs of
the supstrate and the product
C
0:1
C
2:1
C
2:1
Diels-Alder reaction
1:2
C
0:1
C
2:1
C
Mathematical trees grow in
chemistry
• molecular graphs of acyclic compounds
are trees
• example: alkanes
• basic fact about trees: |V| = |E| + 1
• basic fact about graphs: 2|E| = sum of all
vertex degrees
5–isobutyl–3–isopropyl–2,3,7,7,8-pentamethylnonan
•
•
•
•
•
•
•
Alkanes: CnHm
no circuits & no multiple bonds tree
number of vertices: v = n + m
n vertices with degree 4, m vertices wit degree 1
number of edges: e = (4n + m)/2
for every tree e = v – 1
4n + m = 2n + 2m – 2 m = 2n + 2
a formula CnHm represents an alkane only if m =
2n + 2
methane CH4
ethane C2H6
propane C3H8
Topological indices
• properties of substances depend not only of their
chemical composition, but also of the shape of
their molecules
• descriptors of molecular size, shape and branching
• correlations to certain properties of substances
(physical properties, chemical reactivity, biological
activity…)
Wiener index – 1947.
1
W(G)
dij sum of distances between all pairs
2 i, jV ( G )
d(i), d( j) 1
of vertices in a H-supressed graph;
only for trees; developed to
determine parrafine boiling points
Randić index – 1975.
1
Good correlation ability
(G )
d (u )d (v) for many physical &
e {u, v}E
biochem properties
Hosoya index – p(k) is the
number of ways for
|E|/ 2
choosing k non-adjacent
Z(G)
p(k) edges from the graph;
k 0
p(0)=1, p(1)=|E|
topological indices and boiling points of several primary amines
Name
Wiener index
(W)
Randić index
()
Hosoya index
(Z)
Boiling
point/oC (17)
methylamine
1
1
2
-6
ethylamine
4
1,414
3
16,5
n-propylamine
10
1,914
5
49
isopropylamine
9
1,732
4
33
n-butylamine
20
2,414
8
77
isobutylamine
19
2,27
7
69
sec-butylamine
18
2,27
7
63
tert-butylamine
16
2
5
46
n-pentylamine
35
2,914
13
104
isopentylamine
33
2,063
11
96
120
100
80
Bp/C
60
40
20
120
0
0
10
20
30
40
100
W
80
60
Bp/C
40
20
0
140
0
-20
1
2
R
3
4
120
100
80
Bp/C
-20
60
40
20
0
0
-20
5
10
Z
15
• possible exercises for pupils:
• obviously: to compute an index from a given
graph
• to find an expected value of the boiling point of
a primary amine not listed in a table, and
comparing it to an experimental value. Such an
exercise gives the student a perfect view of how
a property of a substance may depend on its
molecular structure
Examples
5
4
2
• 2-methylbutane
3
1
•W=
0,5((1+2+2+3)+(1+1+1+2)+(1+1+2+2)+(1+2+3+3)+
(1+2+2+3)) = 18:
•
1
1
1
1
R
1 3
3 2
2 1
1 3
2,270
• There are four edges, and two ways of
choosing two non adjacent edges so
• Z = p(0) + p(1) + p(2) = 1 + 4 + 2 = 7
For isoprene W isn’t defined, since
5 its
molecular graph isn’t a tree
4
2
Randić index is
1
1
1
1
R
1 3
3 2
and Hosoya index is
Z = 1 + 6 + 6 = 13.
2 1
3
1 3
5
4
2,270
1
3
For cyclohexane 5W isn’t defined, since
5
its molecular
graph isn’t a tree
4
4
2
2
Randić index is
1
R6
3
12 2
and Hosoya index is
Z = 1 + 6 + 18 + 2 = 27.
3
3
2
1
5
1
4
6
3
1
2
Enumeration problems
• historically the first application of graph theory
to chemistry (A. Cayley, 1870ies)
• originally: enumeration of isomers i.e.
compounds with the same empirical formula,
but different line and/or stereochemical formula
• generalization: counting all possible molecules
for a given set of supstituents and determining
the number of isomers for each supstituent
combination (Polya enumeration theorem)
• although there is more combinatorics and group
theory than graph theory in the solution, the
starting point is the molecular graph
Cayley’s enumeration of trees
• 1875. attempted enumeration of isomeric alkanes
CnH2n+2 and alkyl radicals CnH2n+1
• realized the problems are equivalent to
enumeration of trees / rooted trees
• developed a generating function for enumeration
of rooted trees (1 x) A (1 x) A (1 x) A A0 A1 x An1 x n1 ...
• 1881. improved the method
for trees
0
1
n
Pólya enumeration method
• 1937. – systematic method for enumeration
• group theory, combinatorics, graph theory
• cycle index of a permutation group: sum of all cycle
types of elements in the group, divided by the
order of the group
• cycle type of an element is represented by a term of
the form x1ax2bx3c ..., where a is the number of fixed
points (1-cycles), b is the number of transpositions
(2-cycles), c is the number of 3-cycles etc.
• when the symmetry group of a molecule
(considered as a graph) is determined, use the cycle
index of the group and substitute all xi-s with sums
of Ai with A ranging through possible substituents
2
3
1
4
6
Example
5
• how many chlorobenzenes are there? how many
isomers of various sorts?
• consider all possible permutations of vertices
that can hold an H or an Cl atom that result in
isomorphic graphs (generally, symmetries of the
molecular graph that is embedded with respect
to geometrical properties)
• of 6!=720 possible permutations only 12 don’t
change the adjacencies
2
3
1
1 symmetry consisting od 6 1-cycles: 1· x16
4
6
5
2
1
6
3
5
6
4
1
5
2
4
2 symmetries (left and right rotation
for 60°) consisting od 1 6-cycle: 2· x61
3
2 symmetries (left and right rotation
for 120°) consisting od 2 3-cycles: 2· x32
6
5
1
4
2
3
3
2
4
1
5
6
3 symmetries (diagonals as mirrors)
consisting od 2 1-cycles and
2 2-cycles: 3· x12 · x22
4 symmetries (1 rotation
for 180° and 3 mirror-operations with
mirrors = bisectors of oposite pages)
consisting od 3 2-cycles: 4· x23
summing the terms cycle index
1 6
(x1 2x32 4x23 3x12x22 2x61 )
Z(G )
12
substitute xi = Hi + Cli into Z(G)
H6 H5Cl 3H4Cl2 3H3Cl3 3H2Cl4 HCl5 Cl6
i.e. there is only one chlorobenzene with 0, 1,
5 or 6 hydrogen atoms and there are 3
isomers with 4 hydrogen atoms, with 3
hydrogen atoms and with with 2 hydrogen
atoms
Planarity and chirality
• planar graphs: possible to embed into the
plane so that edges meet only in vertices
• a molecule is chiral if it is not congruent to
its mirror image
• topological chirality: there is no
homeomorphism transforming the
molecule into its mirror image
• if the molecule is topologically chiral then
the corresponding graph is non-planar