NIHR ACAT pilot overview - University Of Worcester
advertisement

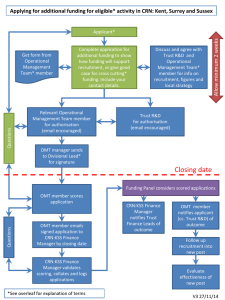
ACAT Pilot Amber O’Malley, CRN Funding and Contracts Manager CRN AcoRD Business Lead Delivering clinical research to make patients, and the NHS, better Session overview • Overview of ACAT Pilot Project – Amber O’Malley • Activity Capture and Attribution Template (ACAT) – Baljit Galsinh • Walkthrough of the ACAT – Karen Hampshire • Q&A AcoRD Implementation AcoRD guidance • Published May 2012 • Applies to new grant applications submitted after 1 October 2012 Primary reasons for change: • Improving the consistency of cost attribution; and • Encouraging more consistent funding of the costs of research https://www.gov.uk/government/publications/guidance-on-attributingthe-costs-of-health-and-social-care-research ACAT Pilot • NIHR Clinical Research Network (CRN) is leading a pilot to support implementation of AcoRD guidance working in partnership with DH, NIHR, AMRC and range of charities 3 Tools and services to be piloted New tool and services designed to help researchers and funders identify and attribute the activities appropriately in research studies in line with AcoRD guidance Activity Capture and Attribution Template (ACAT) • Designed to support researchers to apply the AcoRD guidance to identify and attribute correctly the activities being undertaken as part of a research study • Produce estimated costs of study activities, to give an indication of the resources required to deliver the study. Pre application support service • AcoRD Specialists / NIHR provide support on attribution and independent completion of ACAT • List of AcoRD Specialists available on NIHR CRN website • Other organisations e.g. CTUs, RDSs also provide range of support services ACAT Review service • NIHR CRN / CCF / NETS CC check attribution and resource 4 Aims of the Pilot The purpose of the pilot is to: • Obtain feedback on the usability of the ACAT • Identify any types of research study where the ACAT is inappropriate • Assess the level of training and support that researchers and funders will need to use the ACAT • Assess whether the NIHR CRN AcoRD specialist role meets the needs of researchers • Assess the resource implications for NIHR CRN of rolling out the ACAT and the advice and review process for DH, funders, NIHR CRNs and researchers • Assess whether the review process is likely to deliver the anticipated reduction in delays to study commencement 5 ACAT Pilot Project • Pilot exercise started in December 2013 and due to conclude in the autumn of 2014 • Range of funders participating from AMRC charities and NIHR programmes – Cancer Research UK – Arthritis Research UK – Prostate Cancer UK – Diabetes UK – NIHR • Evaluation exercise commenced and recommendations to DH late 2014 6 UK Wide Working Studies led in Wales • Welsh researchers are included in the ACAT pilot and required to use the ACAT as part of their applications submitted to participating funders • Pre application support and the formal review process for Welsh led studies will be undertaken centrally by the NISCHR AHSC Contract and Costing Service Studies led in Scotland • Researchers based in Scotland are exempt from the ACAT pilot • NHS Research Scotland is developing its own costing system which will provide similar information to the ACAT 7 Feedback and Evaluation • All funders, researchers and AcoRD Specialists participating in the pilot will be asked to provide feedback on their experience of using the associated CRN processes i.e. the ACAT, pre application support and the ACAT Review. • Various mechanisms in place to support evaluation approach • May not be directly involved in the pilot but can also feedback your comments via CRN AcoRD email CRNCC.Acord@nihr.ac.uk 8 Benefits of the ACAT, pre application support service and ACAT Review Completion of the ACAT, the introduction of a new advice and review process will: • Help funders to identify and address any issues regarding attribution and funding at an earlier stage in the funding process • reduce delays to study commencement and • limit the need for funders to make additional resources available part way through a study • Improve the consistency of cost attribution • Support early engagement between researchers and NIHR CRN • enables NIHR CRN to plan resources to support effective delivery • Help funders review value for money of the study by setting out the study’s full resource requirements 9 Learning Resources All learning resources are accessible via the NIHR CRN website: • E-learning attribution tool • DH slide set • CRN slide set • ACAT Briefing sessions • ACAT tutorial (~45 minutes): video format • Visit: http://www.crn.nihr.ac.uk/can-help/fundersacademics/support-for-non-commercial-studies/acord/ • Contact crncc.acord@nihr.ac.uk for queries 10 ACAT Baljit Galsinh CRN Management Accountant Delivering clinical research to make patients, and the NHS, better The Brief • Create a tool that supports the consistent application of AcoRD principles, and estimates the costs of the study • Use Industry Costing Template as a starting point Purpose of the ACAT • To support researchers to apply the AcoRD guidance and correctly attribute the activities being undertaken as part of a research study • To produce estimated costs of these activities, to give an indication of the resources required to deliver the study Purpose of the ACAT • The ACAT is primarily a cost attribution tool • The ACAT is not a comprehensive costing template, and therefore will not dictate the amount of funding paid by the grant funder(s), or the value of support provided by the NIHR Clinical Research Network • Funding applications should still be costed using existing local costing tools • ACAT costings based on activities, not individuals Scope of the ACAT • Research Costs (Part A & B) • NHS Support Costs – but not central management and sponsorship costs • NHS Treatment Costs (the ACAT does not calculate excess treatment costs – these must be calculated separately and entered directly) Source of costs in the ACAT • Costs (and time estimates) from CRN industry costing template (excluding overheads and capacity building element) • The costs within the ACAT are being continually reviewed, in 2014 the investigational costs will be reviewed. • Research (Part A) costs hidden to avoid impression that ACAT dictates funding amount ACAT development • Designed to be as automated as possible • Involved experts from across the NHS • Not expected to capture 100% of studies (may have to use template creatively) • Appropriate balance must be struck between complexity and accessibility ACAT Pilot Activity Capture and Attribution Template (ACAT) Pre-Application Support and Advice Karen Hampshire, Lead RM&G Manager (AcoRD Specialist) CRN: West Midlands 02/07/2014 Delivering clinical research to make patients, and the NHS, better What is the ACAT for Investigators? 1. A tool that supports the consistent application of AcoRD principles and estimates the costs of the study What’s the purpose of the AcoRD Specialists? 1. To support investigators to apply the AcoRD guidance and correctly attribute the activities being undertaken as part of a research study for AMRC funder (not NIHR funding streams) 2. To produce estimated costs of these activities, to give an indication of the resources required to deliver the study at SITE level. What needs to be remembered? 1. ACAT costings based on activities (the primary purpose of the activity), not individuals Reality 1. 2. 3. 4. 5. Don’t assume there is a general understanding of the AcoRD Guidance – understanding the difference between attributions – understanding of Research Part A & Part B costs Received requests for support for NIHR funding streams as well AMRC – Locally we support all funding streams if possible Requested investigators to give us 2 weeks notice before funding submission – Calls for support come late (30mins before the deadline) Contacted mainly by a mixture of CTU’s, Project managers within R&D Departments – No direct contact from CI’s yet Key Questions: – ‘What is an ACAT form?’ – ‘Do I need to complete one of these?’ – ‘Why do I need to complete one of these?’ Assumptions 1. Sent out communications to our local research community - for many it was the first time they have heard about ACAT and even AcoRD! 1. 2. ACAT Pilot on the NIHR CRN website AcoRD Guidance of DH website 2. The ACAT should have been completed by the investigator so should be intuitive 1. 2. This is not always the case as it would have been the first time they would have seen this form Put off by the size of the form which they would have need to complete over and above the funding application Questions from Investigators 1. Can you do my study costings please? 1. Unfortunately not. These need to be done in association with your R&D Departments and Finance Departments. Pre-Application support would not have access to this type of information. Their remit is to support the completion of the template, help identify all the activities and assist in attributing correctly 2. What is the difference between ‘Per Participant costs’ and ‘Study Costs’ 1. ‘Per participants costs’ are study activities which are clearly linked to participants (Questionnaires, investigations, medical history informed consent etc.) 2. ‘Study Costs’ are activities which are generally not linked to participants and occur less frequently during the study Questions from Investigators • The finance calculations in the ACAT are different. Why? – The cost of the activities are taken from the Industry costing template with overheads, capacity building element and MFF stripped out. The costs are national averages and not local costs • Does the completion of the ACAT Template have an effect on my application? – No. This is a pilot and is being run in parallel with the application process. The application process will review the finance information given as part of the application form ACAT Demo 26 Requests for Pre Application Support 1. Should be requested as early as possible 1. Already have some deferred RfPB Sept closing dates 2. Request can come from anywhere 3. Think about completing a ‘Schedule of Events’ 4. Initially email or telephone but should arrange for a face to face meeting with your AcoRD Specialist My experience of Pre Application Support with a research team 1. Initial discussions take 20-30 min to understand the patient pathway for the study 2. Pre Application Support review of the ACAT takes around 60-90 mins depending on the complexity of the study or how well the protocol has been developed. 3. Individual 1:1 support via emails and telephone 10-30 min each after initial meeting. 4. Help is out there! 1. If we are not sure then the AcoRD Specialist have recourse to contact other AcoRD experts. What have we done? AcoRD Specialists (Central) Karen Hampshire Kirsty Hunter ACATs supported 2 x CTAAC 5 x RfPB 1 x CRUK 1 x Care home study (trial only) 9 ACATs submitted ACATs deferred 2 x CTAAC 2 x RfPB 1 x CRUK 5 3 x RfPB Number of training sessions 6 Number trained 71 Organisations trained Network staff, RDS, CTUs, R&D Departments, Universities ACAT information dissemination at events BBC CLRN Showcase Event, 17.03.14 UHBNHSFT Research Showcase Event, 20.05.14 Communication Updates – ‘Fridays Email’ 29 Advice 1. 2. Use the pre-application support service!! Contact an AcoRD Specialist before you have looked at the ACAT if you are new to it 3. Use the ‘HELP’ tab and Guidance section in the ACAT for more detail on attribution 4. Highlight/ list all activities from the grant application 5. Use the automated section as much as possible 6. Use the drop down menus to easily identify all the relevant activities 7. Don’t spend too much time searching for an activity 8. Be aware of potential quirks in the tool (pilot) 9. Don’t be conscious of keeping costs down! 10. Record your feedback to inform the pilot ~ the quirks 30 Benefits 1. Excellent tool for addressing any confusion over attribution 2. Supports the AcoRD guidance and may be used to answer questions not addressed in the AcoRD FAQs 3. Investigators are able to identify potential costs earlier 4. Better understanding of cost attribution all round 5. More confidence in panel members funding study 6. Open and transparent 31 AcoRD Specialist for CRN: West Midlands Central Team Kirsty Hunter Karen Hampshire • Telephone: 0121 627 2843 • Email: cspbbcclrn@uhb.nhs.uk North Team Mary Anne Darby Pam Devall • Telephone: 0845 602 6772 • Email: sch-tr.wmnclrn@nhs.net South Team Katie Williams Rachel Davis • Telephone: 01564 711 711 • Email: uhc-tr.wmsclrn@nhs.net 32