Ferrous and Non-Ferrous Metals
advertisement

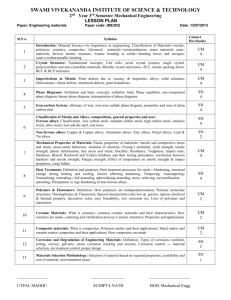
Ferrous and Non-Ferrous Metals Group 5 Josh Agenbroad Joy Best Iris Gallegos Keith Griego September 19, 2005 Ferrous Metals Iron as a base metal Wide Range of Applications -Sheet Steel (cars, aircraft) -Plates (ships, bridges) -Structural (I-Beams) -Machinery (gears, axles, crankshafts) Example: Automobiles are generally 55-60% by weight ferrous metals. Production Coke: produces heat and carbon-dioxide Limestone: acts as Flux removes impurities as slag Iron Ore: Pellets Blast Furnace Steel More refined –less manganese, silicone, carbon Requires higher temperatures Stronger and easier to work with. Electric Furnace High temperatures Heat from electric arc 3500 degrees F Basic Oxygen Furnace Faster output Good for structural components (I-beams) Casting Ingots Molten steel poured into molds (ingots) for cooling to a solid Square, Rectangle, Drum shaped hundreds of pounds to 40tons Reheated for rolling, working Continuous Casting Removes need for ingots Higher quality Reduced cost Carbon and Alloy Steels Carbon and alloy steels are among the most commonly used metals. Are produced in all shapes and sizes depending on the desired application. For example, plumbing fixtures one wants a less corrosive material. Effects of Various Elements in Steels Various elements are added to steels in order to affect their properties like hardness or wear and tear. The higher the percentages the greater the effects. For example Calcium. It deoxidizes steel and improves its toughness. Another element is Carbon. Increasing amounts of carbon reduce toughness and weldability or its ability to transfer heat. Silicon improves strength and corrosion resistance as well as electrical conductivity this is why its used for microprocessors or semiconductors. Residual Elements in Steels What is a residual element? A residual element is an unwanted element which causes undesired affects. Unwanted residual elements: - Antimony, Arsenic, Hydrogen, Nitrogen, Oxygen, and Tin. Cause embrittlement and reduce strength. The way to eliminate the presence of residuals is through refining and processing. Designation for Steels There are a couple different systems used for naming steels based on the percentages of alloying elements and carbon weight. American Iron and Steel Institute (AISI) and the Society of Automotive Engineers (SAE) have designated similar systems. Carbon Steels Carbon steels are categorized in three groups. Lowcarbon containing less than 0.30%, medium-carbons (0.30% to 0.60%), and high-carbon (more than 0.60%). An example of low –carbon is nuts and bolts because they do not require high strength. Medium-carbon is commonly used in machinery because its resistance to high temperatures. High-carbons are unique in that they reduce durability and require heat treatment. Examples are springs, wire, cutlery and cables for the reason that one wants the reduction in ductility. Alloy Steels Alloy steels are steels containing substantial amounts of desired elements. Typically made with more precision than carbon steels. Alloys are used in applications where properties like strength, hardness, creep and fatigue resistance, and toughness are essential. High-Strength Low-Alloy Steels (HSLA) Intended to improve strength to weight ratio of steels. All it means is stronger product but less weight. Ex: earthquake proof buildings. Made just as strong but weigh less. Most commonly used for industrial applications including transportation and construction. A micro alloyed steel is a high strength low alloy steel that is made to eliminate the need for heat treatment. Overall the cost of High-strength low-alloy steels is low. Stainless Steels Simplest idea of a stainless steel, a spoon. A spoon is covered with chromium oxide which protects the metal from corrosion. It builds up again in the effect of the surface being scratched. The higher the carbon content the lower the corrosion resistance. Stainless steel has a lower carbon content and has a higher corrosion resistance. Stainless steel is important because it is used in everyday applications. Stainless Steel Everyday Applications Used for Healthcare and Medical Equipment, and Culinary Tools. Tool and Die Steels Tool and Die steels are alloys designed for high impact and wear resistance used commonly in machining. Nonferrous Metals and Alloys What is a nonferrous metal? What is an alloy? The production methods Important engineering applications The general properties of nonferrous metals Definitions Nonferrous metals- Metals that contain little to no iron Alloys- Base metals combined with other metals or chemicals to enhance the base metals properties Nonferrous metals and alloys are important because they posses important properties such as, corrosion resistance, high thermal and electrical conductivity, low density, and/or ease of fabrication Aluminum and Aluminum Alloys Aluminum was first produced in 1825. It was once considered a precious metal and it was displayed as such in the Paris Exposition of 1855 along side the crown jewels of France. Now very abundant thanks to the electrolytic extraction process. Aluminum has a high strength to weight ratio, resistance to corrosion by many chemicals, high thermal and electrical conductivity non-toxicity, appearance, ease of formability and of machinablity and it is non magnetic Nonferrous Metals in the Real World •Aluminums are designated by 4 numbers followed by a temper designation. Such as 6061-T6 wrought aluminum alloy. •The new Boeing 777 is 70% aluminum Magnesium and Magnesium Alloy First produced in 1808. IS the lightest engineering metal available, with good vibration damping characteristics. Magnesium is an alloying element in various nonferrous metals. Used to strengthen material due to its strong form. Magnesium comes from sea water through electrolysis or by thermal reduction. Copper and Copper Alloys First produced in 4000 B.C. With some of the same properties as Al and it’s alloys. They are the some of the best conductors of electricity and heat, with good corrosion resistance. Copper is produced through a process called Pyrometallurgy. Common alloys of Copper are – – – – Brass Bronze Beryllium copper Phosphor bronze Nickel and Nickel Alloys Discovered in 1751. Like Magnesium it is a major alloying element that imparts strength, toughness, and corrosion resistance. It is also highly magnetic. Nickel is produced by preliminary sedimentary and thermal processes followed by electrolysis. Undersea mining is not yet economical Common alloys of Nickel: – – – – – Nichrome (Nickel Chromium Iron) Invar and Kovar (Nickel Iron) Hastelloy (Nickel Chromium) Monel (Nickel Copper) Inconel (Nickel Chromium) Superalloys Also known as heat resistant alloys or high temperature alloys Good resistance to corrosion, mechanical and thermal fatigue, mechanical and thermal shock, creep, and erosion at elevated temperatures. Max service temperature of 1000 degrees C in structural applications and 1200 degrees C in nonload bearing components. Iron based superalloys, Cobalt based superalloys and Nickel based superalloys are all common. Titanium and Titanium Alloys Discovered in 1791 not produced commercially until 1950. Highly expensive, but posses a very high strength to weight ratio, and corrosion resistance at room and elevated temperatures. Must be handled carefully while being produced to ensure quality of final product. Very extensive process for production which adds to the cost of titanium. Nonferrous Metals Used in the Real World Engine Alliance’s: GP7200 More Nonferrous Metals Beryllium is a hard gray metal that is extracted from the earth, refined and reduced to a very fine powder. It has 6 times the specific stiffness of steel. It is used to make rocket nozzles, space and missile structures, and aircraft disc brakes. Zirconium is a flammable metal and is not found as a metallic. It is silvery in color and is used in electronic components and in nuclear-power reactor applications because of its low neutron absorption. Low-Melting Alloys Lead is a bluish-white lustrous metal. It is very soft, highly malleable, ductile, and a relatively poor conductor of electricity. It is very resistant to corrosion but tarnishes upon exposure to air. Lead pipes of Roman emperors, used as drains from the baths, Lead plumbing pipes from the Roman Empire are still in use. Zinc, is a bluish-white color and is the metal fourth most utilized industrially, after iron, aluminum, and copper. It has two major uses: 1) Galvanizing iron, steel sheet, and wire and 2) as an alloy base for casting. Tin- Known since ancient times, tin is a silvery-white, lustrous, malleabe ductile metal. As a pure metal, tin is used in the production of packaging for food and distilled water, beer and carbonated drinks. It can still be used in storage tanks for pharmaceutical chemical solutions, in capacitors electrodes, fuse wires, ammunitions, sweets or tobacco. Precious Metals Gold is soft and ductile and has good corrosion resistance at any temperature. Typical applications include jewelry, coinage, reflectors, gold leaf for decoration purposes, dental work, electroplating, and electrical contacts and terminals. Silver is a ductile metal and has the highest electrical and thermal conductivity of any metal. However, it develops an oxide film that affects its surface characteristics and appearance. Typical applications for silver include tableware, jewelry, coinage, electroplating, photographic film, electrical contacts, bearing linings, and food and chemical equipment. Platinum is a soft, ductile, grayish-white metal that has good corrosion resistance even at elevated temperatures. Platinum alloys are used as electrical contacts, for spark plugs, as catalysts for automobile pollution-control devices, in filaments, in nozzles, in dies for extruding glass fibers, as jewelry, and in dental work. Shape-Memory Alloys What is a shape memory alloy? Shape memory alloys are metals that exhibit shape memory properties. It allows materials possessing shape memory properties to return to their original shape after having suffered some form of deformation after they are heated to temperatures above their transformation temperature. In most shape memory alloys, a temperature change of only about 10°C is necessary to initiate this phase change. The medical and aerospace and marine industries are the largest consumers of shape memory components The shape memory effect is observed when the temperature of a piece of shape memory alloy is cooled to below the temperature at which the Martensite phases finishes forming . At this stage the alloy is completely composed of Martensite which can be easily deformed. After distorting the SMA the original shape can be recovered simply by heating the wire above the temperature at which the Austenite phase finishes forming. The heat transferred to the wire is the power driving the molecular rearrangement of the alloy, similar to heat melting ice into water, but the alloy remains solid. The deformed Martensite is now transformed to the cubic Austenite phase, which is configured in the original shape of the wire. Shape-Memory Alloys Graph Metal Foams Metal foams are material structures where the metal consists of only 5 to 20% of the structure’s volume. They are light and stiff, they have good energy-absorbing characteristics, making them good for crash-protection and packaging. They have attractive heat-transfer properties used to cool electronic equipment and as heat exchangers in engines. Because they are very lightweight they have been used more now in modern day aerospace applications. Nanomaterials The composition of a nanomaterial can be any combination of chemical elements. Among the current and potential applications for nonomaterials are the following: Flat panel displays for laptop computers and televisions, spark plugs, igniters and fuels for rockets, medical implants and high power magnets. References Kalpakjian, Serope, and Schmid, Steven R. Manufacturing Engineering and Technology. Prentice-Hall, Fifth Edition. Lindbeck, John R. Product Design and Manufacturing. Prentice-Hall, 1995 http://geae.com http://boeing.com