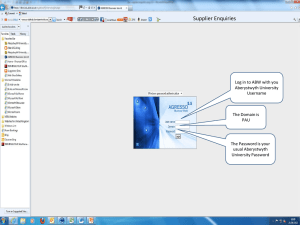
To facilitate the creation of a valid order for OCPO

10
11
12
13
14
15
16
17
1
4
5
2
3
8
9
6
7
Use Case and Functional Requirements Development for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Appendix G: Oxygen Concentrators and Portable Oxygen (OCPO) User Story
Note: This user story is based on Medicare National Coverage Determination (NCD), Local Coverage
Determination (LCD) and CMS Medicare regulations and guidelines. While other payers have participated in the workgroup effort, we have not received specific coverage or workflow requirements from any payer group other than CMS Medicare Fee-For-Service (FFS). If a payer’s coverage requirements necessitate a change in this user story, then a new version of the user story should be created, explicitly identifying the changes and provisions for additional, alternative, requirement-based workflows.
G.1 In Scope
This appendix focuses on defining the user story for oxygen concentrators and portable oxygen, based on the electronic Determination of Coverage (eDoC) General Use Case. Special attention is paid to additional constraints and identification of gaps on and in the general Use Case, as required by the user story. It reuses existing S&I Initiative efforts where possible in the process of selecting standards for structured data documentation requirements, exchange templates, interactive information capture templates, decision support rules and exchange standards. For the immediate effort to determine documentation requirements, the user story will:
24
25
26
27
28
29
18
19
20
21
22
23
30
31
A.
Define required and supporting documentation consistent with clinical best practice and payer policy for oxygen concentrators and portable oxygen (OCPO)
B.
Define providers required/allowed to complete the documentation
C.
Define required and supporting documentation for OCPO
D.
Specify requirements for OCPO documentation
E.
Define order(s) for OCPO
F.
Define certification documentation for OCPO
G.
Define Re-certification documentation for OCPO equipment
H.
Define requirements for information exchange between and among participating entities
(Including workflow required for the ACA-specified OCPO equipment and an optional workflow for non-ACA-specified OCPO equipment)
I.
Define order validation transaction
G.2 Out of Scope
The user story will not address:
32
33
34
A.
Deployment of an electronic service or form that interacts directly with the participating entities
B.
Patient interaction with Payer for OCPO
G.3 User Story Assumptions
35
36
A.
This user story addresses the data set requirements for determination of coverage for OCPO durable medical equipment (DME)
1
37
38
39
40
41
42
B.
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Multiple valid diagnostic, documentation, and order workflows currently exist and include the beneficiary, physician, DME Supplier and potentially other licensed providers and service suppliers.
G.4 Pre-Conditions
The pre-conditions section describes the state of the system, from a technical perspective, that will need to be in place prior to execution of the operation, process activity or task.
43
44
45
46
No additional pre-conditions for OCPO user story
G.5 Post Conditions
The Post Conditions section describes the state of the system, from a technical perspective, that will result after the execution of the operation, process activity or task.
47
48
49
50
51
52
53
Payer Contractor information system processes the submitted documentation for order validation, prior authorization, prepayment review or post payment audit for determination of coverage
The ordering provider and DME supplier systems must retain the validated order, as well as all required documentation (such as the CMN) as part of the record of the provider’s order for the service, as per payer policy or relevant record retention requirements, whichever is longer.
G.6 OCPO Scenario Background (Medicare FFS)
54
55
56
57
58
59
60
The dispensing and use of Oxygen Concentrators and Portable Oxygen (OCPO) is a covered service under
Medicare Part B benefits. Coverage for OCPO is determined reasonable and necessary for beneficiaries with significant hypoxemia who also meet the medical documentation, laboratory evidence, and health conditions as specified by policy. A 2006 Office of the Inspector General (OIG) report titled, “Medicare
Home Oxygen Equipment: Cost and Servicing” reported that in 2004, home oxygen equipment accounted for 24% ($2.7 billion of $11.1 billion) of all Medicare spending for DME, prosthetics, orthotics and supplies.
61
62
63
64
65
66
67
68
69
70
An oxygen concentrator is a device which concentrates the oxygen from a gas supply (typically ambient air) to deliver an oxygen enriched gas mixture. Oxygen concentrators typically use pressure swing adsorption technology and are used very widely for oxygen provision in healthcare applications, especially where liquid or pressurized oxygen is too dangerous or inconvenient, such as in homes or in portable clinics. Oxygen concentrators can also be found in a portable form. Portable oxygen concentrators (or POC) are a portable device used to provide oxygen therapy to patients at substantially higher oxygen concentrations than the levels of ambient air. It is very similar to a home oxygen concentrator, but is smaller in size and more mobile. The portable oxygen concentrator makes it easy for patients to travel freely, as they are small enough to fit in a car and many concentrators are now FAAapproved.
71
72
Certain conditions warrant the use and coverage of oxygen therapy including severe lung diseases such as chronic obstructive pulmonary disease, diffuse interstitial lung disease, cystic fibrosis, bronchiectasis,
4/17/2020 2
73
74
75
76
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story and widespread pulmonary neoplasm. Other conditions requiring the use of oxygen therapy may include hypoxia-related symptoms or findings expected to improve with oxygen therapy such as pulmonary hypertension, recurring congestive heart failure due to chronic cor pulmonale, erythrocytosis, impairment of the cognitive processes, nocturnal restlessness, and morning headache.
77
78
79
80
81
82
83
84
85
86
87
The results of an arterial blood gas or pulse oximetry test provide required supporting evidence for the initial and ongoing need for OCPO.
When oxygen is covered based on an oximetry study obtained during exercise, there must be documentation of 3 oximetry studies in the beneficiary’s medical record: 1) testing at rest without oxygen, 2) testing during exercise without oxygen, and 3) testing during exercise with oxygen applied (to demonstrate the improvement of the hypoxemia) are required. All 3 tests must be performed within the same testing session.
A Certification of Medical Need (CMN) for oxygen equipment must include results of specific testing before coverage can be determined. Suppliers that bill electronically may transmit initial, revised, and recertification CMNs by electronic media using CMSestablished standard formats. Information transmitted from a revised or recertification form CMS-484 must accompany the first claim for monthly benefits submitted after the supplier has received the hard copy form CMS-484 from the certifying physician.
88
89
90
91
In order to establish the necessity of oxygen therapy, a certification from a certifying physician must be provided. An initial, recertification, or revised certification of medical necessity (CMN) must be obtained and submitted by the DME supplier providing the oxygen device to the beneficiary. The certifying physician must document testing and visit requirements for the CMN as outlined by policy.
92 A CMN is required:
97
98
99
100
93
94
95
96
101
102
103
1.
With the first claim for home oxygen, (even if the beneficiary was on oxygen prior to Medicare eligibility or oxygen was initially covered by a Medicare HMO).
2.
During the first 36 months of the rental period, when there has been a change in the beneficiary’s condition that has caused a break in medical necessity of at least 60 days plus whatever days remain in the rental month during which the need for oxygen ended.
1
3.
When the equipment is replaced because the reasonable useful lifetime of prior equipment has been reached.
4.
When the equipment is replaced because of irreparable damage, theft, or loss of the originally dispensed equipment. a.
Irreparable damage refers to a specific accident or to a natural disaster [e.g., fire, flood] b.
Irreparable damage does not refer to wear and tear over time
104 Recertification CMN is required:
105
106
1.
12 months after Initial Certification, (i.e., with the thirteenth month’s claim) for Group I (see definition for Group I below)
1 Refer to the Policy Article NONMEDICAL NECESSITY COVERAGE AND PAYMENT RULES for additional information
4/17/2020 3
107
108
109
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
2.
3 months after Initial Certification, (i.e., with the fourth month’s claim) for Group II (see definition for Group II below)
4/17/2020 4
110
111
112
113
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Group I and II Criteria are outlined in the tables below:
Table 1: Group I Criteria
Patient on Room Air at Rest Patient Tested During Exercise Patient Tested During Sleep
While awake:
Arterial oxygen saturation at or below
88%; or
Arterial Partial Pressure of Oxygen (PO
2
) at or below 55 mm Hg.
Test 1:
Room air at rest arterial saturation results above
56 mm Hg or an arterial oxygen saturation at or above 89%; and
Test 2:
Arterial desaturation during ambulation, without oxygen, falls to or below 55 mm Hg or an arterial oxygen desaturation at or below
88%; and
Test 3:
Documented improvement of hypoxemia during ambulation with oxygen.
If arterial PO
2
at or above 56 mm
Hg or an arterial oxygen saturation at or above 89% while awake, additional testing during
sleep must show: a.
Arterial PO
2
at or below
55 mm Hg, or an arterial oxygen saturation at or below 88%, for at least 5 minutes taken during sleep; or b.
Decrease in arterial PO
2 of more than 10 mm Hg, or a decrease in arterial oxygen saturation more than 5%, for at least 5 minutes, taken during sleep associated with symptoms or signs reasonably attributable to hypoxemia.
Table 1: Group II Criteria
Patient on Room Air at Rest
While awake:
Arterial oxygen saturation of 89%; or
Arterial PO
2
of 56-59 mm
Hg; and a.
Dependent edema suggesting congestive heart failure; b.
Pulmonary hypertension or cor pulmonale, determined by measurement of pulmonary artery
Patient Tested During Exercise Patient Tested During Sleep
Arterial oxygen saturation of 89%; or
Arterial PO
2
of 56-59 mm
Hg; and
Arterial oxygen saturation of 89%; or
Arterial PO
2
Hg; and
of 56-59 mm a.
Dependent edema suggesting congestive heart failure; b.
Pulmonary hypertension or cor pulmonale, determined by measurement of a.
Dependent edema suggesting congestive heart failure; b.
Pulmonary hypertension or cor pulmonale, determined by measurement of
4/17/2020 5
c.
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story pressure, gated blood pulmonary artery pulmonary artery pool scan, echocardiogram, or “P” pulmonale on electrocardiography
(EKG) (P wave greater than 3 mm in standard leads II, III, or AVF); or
Erythrocythemia with a hematocrit above 56%. pressure, gated blood pool scan, echocardiogram, or
“P” pulmonale on
EKG (P wave greater than 3 mm in standard leads II, III, or AVF); or
c.
Erythrocythemia with a hematocrit above 56% pressure, gated blood pool scan, echocardiogram, or
“P” pulmonale on
EKG (P wave greater than 3 mm in standard leads II, III, or AVF ); or c.
Erythrocythemia with a hematocrit above 56%
114
115
There are additional specific requirements based on the initial CMN for Group I and II criteria which include:
116
117
118
119
120
1. The blood gas study must be the most recent study obtained within 30 days prior to the initial date.
For Group I criteria, there is an exception to the 30-day test requirement for beneficiaries who were started on Oxygen while enrolled in a Medicare HMO and transition to fee-for-service Medicare. For those beneficiaries, the blood gas supply does not have to be obtained 30 days prior to the initial date, but must be the most recent qualifying test obtained while in the HMO.
121
122
2. The beneficiary must be seen and evaluated by the treating physician within 30 days prior to the date of initial certification.
123
124
3. Medicare policy may define exceptions to the above timing requirements based on transitions from specific care settings to the home environment (See appropriate policy for specifics).
125
126
127
128
129
130
131
In reviewing a claim and the supporting data, payers compare certain items, especially pertinent dates of treatment. For example, the start date of home oxygen coverage cannot precede the date of prescription or the date of the test(s) whose results establish that the special coverage criteria are met.
Medicare will only cover items when it is in use by a patient. For Medicare to continue coverage for
OCPO DME, patients should need and use the equipment, and a physician must regularly document the patients continued medical need for, and use of, oxygen equipment through the patient’s medical records, as per payer policy.
132
133
134
135
136
137
138
139
This user story envisions an electronic order validation method that allows the ordering provider or the supplier to confirm essential elements of an order for specific services/devices prior to either the delivery of the service or submission of a claim for payment for the services/devices. The goal is to reduce the errors related to insufficient information with regard to the provider, beneficiary, supplier, service/device, and medical necessity.
G.6.1 Oxygen Concentrator and Portable Oxygen User Story
The provider has a visit with the beneficiary who may have a severe lung disease or hypoxia-related symptoms to evaluate and assess their need for an oxygen concentrator or portable oxygen (e.g. -
4/17/2020 6
153
154
155
156
170
171
172
173
174
175
164
165
166
167
168
169
176
177
157
158
159
160
161
162
163
146
147
148
149
150
151
140
141
142
143
144
145
152
178
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
HCPCS codes E0433 and E1390). The provider evaluates and assesses the beneficiary, making use of all available beneficiary/provider interaction documentation. Relevant information may include the history, physical examination, hospital admission and discharge records, diagnostic tests, summary of findings, diagnoses, and treatment plans, as well as office records, hospital records, nursing home records, home health agency records, and records from other healthcare professionals. The provider documents their findings regarding the beneficiary’s visit within the EHR system, including findings regarding the blood gas study (either a pulse oximetry test or arterial blood gas (ABG) test). The provider, if not conducting the blood gas study in-person themselves, sends the signed order/referral requesting the blood gas study be conducted by another qualified provider or supplier of laboratory services. The qualified provider or laboratory service supplier performs the blood gas study and documents the test results within their EHR/LIS (Laboratory Information System) system, and signs and dates the entry. The qualified provider or laboratory service then sends the result of the blood gas study back to the requesting provider.
The provider receives and reviews the test results and determines that OCPO DME is reasonable and necessary based on test results. The provider evaluation and management services are documented in the medical record, which provides the information necessary to complete a Certification of Medical
Necessity (CMN) (CMS Form 484).
ACA 6407 requires the supplier to receive a written order (DWO/CMN) and face-to-face documentation prior to delivery of OCPO equipment for the HCPCS codes specified in the table contained in the Policy
Specific Documentation Requirements Section within the LCD. The LCD states, “A DWO is required prior to delivery of OCPO Systems. The CMN may serve as a DWO, in which case the CMN needs to be received by the supplier prior to the delivery of the OCPO Systems (as listed in the ACA). A CMN is required prior to billing but the DWO/CMN can act as the order or the dispensing order. If the CMN is not being completed as a DWO, then the CMN simply needs to be completed and on file prior to submission of the claim.” If the provider creates or generates the CMN/DWO, they must complete
Sections ‘A’ and ‘B’ of the CMN/DWO before sending it to the DME Supplier. The Supplier receives the documentation, and completes Section ‘C’ of the DWO/CMN before sending it back to the Provider/NPP, who completes Section ‘B’ and signs and dates Section ‘D’ of the DWO/CMN (the above mentioned workflow is required for the exchange of a CMN but optional for the exchange of a DWO). Alternately, the Supplier can also create/generate the CMN/DWO, but they must complete Sections ‘A’ and ‘C’ of the
DWO/CMN before returning the documentation to the Provider/NPP. The Provider/NPP then completes
Section ‘B’ and signs and dates Section ‘D’ of the DWO/CMN before sending it to the DME Supplier. Prior to signing, the order information may optionally be sent for order validation by the Provider/NPP. The
Order Validation Number should be included with the DWO/CMN when it is sent to the supplier. The
DME supplier receives, reviews, and processes the documentation, and upon receipt, the order information may optionally be sent by the supplier for order validation. The DME supplier provides the device to the beneficiary, and the beneficiary signs off on receiving the device within the Proof of
Delivery (POD) documentation. Proof of Delivery documentation must contain: a) Beneficiary’s name
4/17/2020 7
190
191
192
193
194
195
196
197
198
199
200
179
180
181
182
183
184
185
186
187
188
189
206
207
208
209
210
211
212
213
214
215
201
202
203
204
205
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story b) Delivery address c) Package identification number (linking supplier’s delivery documentation with delivery service records) d) Quantity Delivered e) Detailed descriptions of item(s) f) Manufacturer (where required) g) Serial Number/Lot Number (where required) h) Delivery Date i) Signature of person accepting delivery j) Relationship to beneficiary k) Signature date
In the alternative user story flow for OCPO orders, which do not contain items specified in the ACA, the
Provider evaluates and assesses the beneficiary, and determines if the patient is eligible for OCPO. After updating the beneficiary record, the Provider creates/generates a dispensing order, as per payer policy.
The Dispensing Order must contain the following items: a) Description of the item b) Beneficiary’s name c) Prescribing physician’s name d) Date of the order (for verbal orders, use the date the supplier is contacted by the physician) e) Start date (if the start date is different than the date of the order) f) Physician signature (if written order) g) Supplier Signature (if verbal order)
The Provider sends the dispensing order for OCPO DME (including optional order validation number if applicable) to the DME Supplier. The DME Supplier receives and reviews the dispensing order. The
Provider/NPP then creates/generates the DWO/CMN documentation and follows the same document exchange sequence as outlined in the workflow required for ACA OCPO equipment. The DWO must contain: a) Beneficiary’s name b) Physician’s name c) Date of the order d) Start date (if different than date of order) e) Detailed description of the item(s) (adhering to requirements as per LCD) f) The prescribing practitioner's National Provider Identifier (NPI) g) Physician signature and signature date h) The means of oxygen delivery (cannula, mask, etc.) i) The flow rate j) Frequency of use
4/17/2020 8
240
241
242
243
244
245
246
247
248
249
250
251
252
222
223
224
225
226
227
228
229
230
216
217
218
219
220
221
231
232
233
234
235
236
237
238
239
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
The provider processes, signs, and dates all documentation received by the DME Supplier, including the
DWO/CMN, and dispensing order, without which payment will be denied by the Payer. The Provider then proceeds to send all of the completed documentation to the DME supplier within the time-period specified by the payer. The DME supplier receives, reviews, and processes the documentation. The DME supplier provides the device to the beneficiary, and the beneficiary signs off on receiving the device within the Proof of Delivery (POD) documentation.
The DME supplier prepares and submits the signed documentation, including the CMN, DWO, and POD, for payment for the OCPO DME to the payer, including the Order Validation Number if one exists. If prepayment review is required, the DME Supplier submits supporting documentation prior to payment. The payer receives the signed documentation and pays the DME supplier. The payer conducts a postpayment audit and requests documentation from the DME Supplier. The DME Supplier prepares, signs, dates, and submits all required documentation to the Payer for review. The payer receives and processes the signed documentation and responds to the DME supplier. (Best clinical practice indicates the DME supplier should notify the provider(s) on delivery and acceptance of the OCPO DME.
Transactions involved with this best practice are out of scope.)
To facilitate the creation of a valid order for OCPO this user story introduces the concept of an optional order validation method that involves communication of specific order elements between the Provider or DME Supplier and the responsible Payer. It is the expectation that this validation process will enable the Payer to verify order elements that are transmitted as part of the validation process. The Payer will respond to the order validation request by identifying errors if they exist, or, if the order elements are successfully verified, provide a unique “order validation number” for the order that may be included in the claim submission. Based on this concept, the Provider and/or DME Supplier may send elements of the dispensing order and/or the DWO to the Payer for order validation. Order validation may occur at any of four points in the OCPO workflow:
1.
Prior to the Provider issuing the dispensing order for OCPO DME to the Supplier (Alternate
Workflow for non-ACA specified OCPO equipment)
2.
After the DME Supplier receives the dispensing order for OCPO DME from the Provider and before they dispense the OCPO DME (Alternate Workflow for non-ACA specified OCPO equipment)
3.
After the Provider/NPP completes and signs the DWO/CMN (ACA Specified OCPO equipment)
4.
After the Supplier receives the signed and dated DWO/CMN/Progress Note (if required by
Supplier) from the Provider (ACA Specified OCPO equipment)
The Payer receives and validates the elements of the dispensing order and/or the DWO. The Payer responds to the order validation request, and if successful, returns an order validation number to the
Provider or DME Supplier. The Provider or DME Supplier receives the “order validation number” if validation is successful. For a successful response the DME supplier will include the order validation number in the claim submission.
4/17/2020 9
253
254
255
256
257
258
259
260
261
262
263
264
265
266
267
268
269
270
271
272
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Order Validation Error Response
If order validation fails, then the payer will return an error response indicating the order elements that failed validation. The Provider may make the appropriate corrections to the order and either the
Provider or DME Supplier may resubmit the elements of the dispensing order and/or the DWO for order validation.
Additional Scenarios That May Require a New Order for OCPO (see Payer policy)Break in medical necessity of over 60 days
Beneficiary moving to another region out of the suppliers’ service area
Prescription for flow rate changes based on ABG or pulse oximetry tests
Change from stationary O2 to portable O2 (and vice versa)
New treating physicians
Recertification for continued medical need past 36 months
Length of need expiration
Change in supplier for OCPO
Equipment replacement
Equipment replacement post reasonable useful lifetime
G.6.2 Medicare FFS - Specific OCPO Requirements
The corresponding LCD policy for Oxygen Concentrators and Portable Oxygen (OCPO) is included in the attached document below:
273
274
275
276
277
278
279
280
G.6.3 Sequence Diagram
A Sequence Diagram is primarily used to show the interactions between objects in the sequential order that they occur. This representation can make it easy to communicate how the exchange works by displaying how the different components interact. The primary use of the diagram is in the transition from requirements expressed as use cases to the next and more formal level of refinement.
4/17/2020 10
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
281
4/17/2020 11
282
283
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Figure 1 - Workflow for ACA Specified OCPO Equipment (Sequence Diagram)
4/17/2020 12
284
285
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Provider/NPP
EHR System
Qualifying Provider/Laboratory
EHR System
DME Supplier
Information System
Payer/Payer Contractor Information
System
Beneficiary
1. Evaluate &
Assess Beneficiary
2A. Perform O2 Saturation Study,
Document, and Sign Result
Alt: 2B1. Send Signed Order
Requesting ABG Test
2B4. Send Signed, Documented Results
2B5. Receive Signed
ABG Test Results
2B6. Review & Co-Sign
ABG Test Results
3. Update Beneficiary Record,
Including evaluation and management services
2B2. Receive Signed
Order
2B3. Document, and Sign
ABG Test Results
4. Create/Generate Dispensing Order
Alt: 5. Send Order Information for Order Validation
5A. Receive Order Validation Request
Alt: 5D1. Payer Response Includes Order
Validation Number
Alt: 5D1a. Receives Order Validation Including Order Validation number
Alt: 5D2. Payer Response Includes Error Notification
Alt: 5D2a. Receives Order Validation Response Including Error notification
6. Send Dispensing Order for OCPO DME (Including Optional Order Validation Number)
8E. Send Error Notification Response for Order Modification
8F. Receive Error Notification Response from Supplier
5D. Payer Response
5B. Review Dispensing Order/DWO
5C. Generate response to Order Validation Request
7. Receives and Reviews
Dispensing Order
Alt: 8. Send Order Information for Order Validation
8A. Receive Order Validation Request
Alt: 8D1. Payer Response Includes Order
Validation Number
8B. Review Dispensing Order/DWO
8C. Generate response to Order
8D. Payer Response
Validation Request
Alt: 8D1a. Receives Order Validation Including Order Validation number
Alt: 8D2. Payer Response Includes Error Notification
Alt: 8D2a. Receives Order Validation Response Including Error notification
9. Dispense OCPO DME to Beneficiary
10. Beneficiary Receives OCPO DME
11. Beneficiary Signs POD
12. Beneficiary Returns POD to Supplier
13. Receives POD
14A. Creates/Generates DWO/CMN (can be used as DWO)
14A1. Sends DWO/CMN prior to delivery of OCPO With Section ‘A’ and ‘B’ Completed
14B. Alt: Creates/Generates DWO/CMN and completes
Sections ‘A’ and ‘C’
14A2. Receives DWO/CMN with Sections ‘A’ and ‘B’ Completed
14A3. Completes Section C of DWO/CMN
14A4. Returns DWO/CMN Documentation to Provider/NPP with section ‘A’ , ‘B’, ‘C’
14B1. Returns DWO/CMN Documentation to
Provider/NPP with sections ‘A’ and ‘C’ completed
14A5. Receives DWO/CMN
14B2. Completes Section ‘B’ of DWO/CMN
15. Signs Section ‘D’ of DWO/CMN
16. Sends All completed documentation back to the Supplier
17. Receives all completed documentation
18. Prepares Documentation for Payment
19. Maintains all documentation regarding
OCPO DME for Respective Beneficiary
20. Send claim, CMN, and optional order validation number for payment
(and other supporting documents if pre-payment review is required)
Provider/NPP
EHR System
Qualifying Provider/Laboratory
EHR System
23. Receive and Processes Payment
24. Conducts Post-Payment Audit
25. Sends Request for Documentation
Regarding Post-Payment Audit
26. Receives and Reviews Request for Documentation
27. Send All Signed and Supported Documentation
28. Receive &
Process Signed
Documentation
29. Send Response
30. Receive &
Process Response
DME Supplier
Information System
Payer/Payer Contractor Information
System
Beneficiary
4/17/2020 13
286
287
288
289
297
298
299
300
301
290
291
292
293
294
295
296
302
Use Case Development and Functional Requirements for Interoperability
Electronic Determination of Coverage (eDoC) Use Case
Oxygen Concentrators and Portable Oxygen User Story
Figure 2 - Workflow for Non-ACA Specified OCPO Equipment (Sequence Diagram)
The following document maps each of the inter-system steps in the Sequence Diagram to a particular document or document set exchanged between systems as part of this user story. The following notation is used for the artifacts:
XNY
•
X=Source
N= Document number
Y= Signature, where A indicates a document without a co-signature and B indicates a document with a co-signature
If a document is co-signed, the original document does not need to be included. If
N changes, all versions of N should be included.
An Artifact can either be one or more CDA compliant documents
G.7 Dataset Requirements
This table lists the data elements and data element sets that will be available within the documentation including the Face to Face Encounter, signed and dated Certification, signed and dated Plan of Care, and documentation bundle. Historically, the optional/required nature of each data element is deferred to the discussions during the harmonization phase of the Initiative; however, some data elements do contain recommendations for optionality. Each data element listed below is necessary for some aspect of the User Story; however, the table does not specify exactly how they may be used together.
The final Dataset Requirements list has been provided in a spreadsheet format and is linked/embedded below. If you have any difficulty opening this file, you may also find a link to the same on the esMD eDoC
Wiki ( http://wiki.siframework.org/esMD+-+Electronic+Determination+of+Coverage ). Elements within the Dataset Requirements have been mapped to the artifacts established as part of the Sequence
Diagram analysis referred to within the Sequence Diagram portion of this user story.
303
304
305 esMD OCPO Data
Set Requirements
4/17/2020 14