Document
advertisement

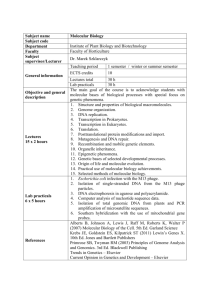
MOLECULAR BIOLOGY – DNA replication, transcription DNA REPLICATION (semi-conservative method) MOLECULAR BIOLOGY – DNA replication, transcription Meselson-Stahl experiment REPLICATION FORK 1000 nt / sec ! The enzyme DNA polymerase uses the two parental strands as a template to faithfully synthesise new daughter strands according to the specific Watson & Crick base-pairing system (A-T and G-C) Figure 5-2 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Semi-conservative replication by DNA polymerase requires that the two anti-parallel parental DNA strands are unwound to give a single stranded templates Unwinding and strand separation achieved by DNA helicase using the enegy released from ATP hydrolysis Figure 5-14 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Secondary structure in the single stranded template can hinder DNApol Single-strand binding proteins facilitate DNApol’s progress Figure 5-16 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Newly synthesised strand replicated in a 5’ - 3’ direction New dNTPs are added to the free 3’OH group of preceding nucleotides in the DNA strand by means of a condensation reaction thus forming a new phosphodiester bond Pyrophosphate and water are byproducts Incoming deoxyribonucleotide trisphosphates or dNTPs (i.e. dATP, dCTP, dTTP or dGTP depending on base pairing with TEMPLATE STRAND) Figure 5-3 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Methotrexate Synthesis of dNTP substrates required for DNA replication anti-cancer drug Folate cofactor only obtainable as a dietary supplement (extra supplements for children and pregnant women) dTDP UDP ADP GDP CDP deoxyUDP deoxyADP deoxyGDP deoxyCDP ribonucleotide reductase dTTP dATP dGTP dCTP kinase MOLECULAR BIOLOGY – DNA replication, transcription Chromosomal DNA synthesis catalysed by DNA polymerase III (DNApol III) DNApol III requires the help of ‘sliding clamp’ in order to bind DNA and start replication The sliding clamp however requires a complex of proteins (i.e. the ‘clamp loading complex’) plus the energy released from ATP hydrolysis to be loaded onto the DNA DNA synthesis can now occur in 5’ 3’ direction although any basepairing mistakes can be corrected by removing the incorrect base via the ‘3’-exonuclease activity’ of the DNApol III complex DNApol III Figure 5-18c Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription DNA polymerase synthesizes in 5’ 3’ direction therefore the two newly synthesized daughter strands are made differently replication direction 3’ 5’ leading strand lagging strand 3’ 5’ 3’ 3’ 5’ 5’ 5’ 3’ Okazaki fragmentsare eventually joined (ligated) together to form a complete strand MOLECULAR BIOLOGY – DNA replication, transcription How does DNA synthesis get started? DNA polymerase III can not simply start synthesizing a new strand It can only elongate from existing one (i.e. a free 3’OH group is required) A specialized RNA polymerase called ‘DNA primase’ can simply start the synthesis of a new strand using the template DNA strand as a guide These 11-12 nucleotide RNA primers then provide the free 3’OH required by DNApol III to replicate the rest of the DNA Figure 5-11 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription What happens to the RNA primers and Okazaki fragments on the lagging strand? DNApol III completes the synthesis of the DNA Okazaki fragment up until the previous RNA primer without joining the two molecules together The ‘gap’ between the Okazaki DNA fragment and the RNA primer is recognised by ‘DNA polymerase I’ that then removes the RNA primer and fills in the space with template directed DNA DNApol I Lastly the two adjacent DNA Okazaki fragments are joined by the enzyme ‘DNA ligase’ Figure 5-12 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription DNA Replication Summary N.B. the lagging strand template is bent round so both DNApol’s proceed in the same direction! Figure 5-19a Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Detailed electronmicrograph of a bacterial DNA replication fork Figure 5-19b,c Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Electronmicrograph of two replication forks progressing around a circular bacterial DNA genome Figure 5-6 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Summary of bidirectional replication forks with leading and lagging strand synthesis Figure 5-25 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription The action of helicase at the replication fork places great strain on the DNA double helix ahead of it because the two ends of the helix cannot freely rotate with respect to each other Figure 5-21 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription An enzyme called ‘DNA topisomerase I’ relieves this strain by catalysing a break in the phosphodiester backbone of one DNA strand allowing the two ends of the helix to rotate relative to each other Figure 5-22 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription DNA replication summary http://highered.mcgraw-hill.com/sites/0072556781/student_view0/chapter11/animation_quiz_2.html MOLECULAR BIOLOGY – DNA replication, transcription Known as OriC in E-coli. N.B. bi-directional replication from a single replication origin. Relatively small bacterial genomes are circular and are usually replicated from a single ‘replication origin’ that consists of tandem repeat rich DNA sequences Figure 5-26 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Large eukaryotic genomes (multiple linear chromosomes) are replicated from many different ‘origins of replication’ • Multiple origins of replication comprising many different sequence variations (approx 100 000 in human genome) • Not all activated at the same time • Mechanism involves the assembly of the ‘pre-replication complex (pre-RC)’ of proteins prior to their activation and initiation of DNA synthesis Figure 5-34 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription During DNA replication there is aProblem with the end of the lagging strand: ... progressive shortening of chromosomal ends and genetic instability MOLECULAR BIOLOGY – DNA replication, transcription Chromosome ends comprised of TELOMERES Telomeres made of many repeats (TTAGGG)20-HUNDREDS TTAGGGTTAGGGTTAGGGTTAGGG Species Repeat Sequence Arabidopsis TTTAGGG Human TTAGGG Oxytricha TTTTGGGG Slime Mold TAGGG Tetrahymena TTGGGG Trypanosome TAGGG Yeast (TG)1-3TG2-3 Telomeres provide a kind of ‘buffer’ for the chromosomal ends that protect genes located in the ‘sub telomeric’ regions Therefore only telomeric sequence is lost during DNA replication HOW ARE TELOMERES MAINTAINED? MOLECULAR BIOLOGY – DNA replication, transcription TELOMERES ARE ELONGATED BY ACTION OF TELOMERASE Conventional DNA synthesis is achieved by RNA priming of the elongated parental strand thus maintaining telomere length and chromosome integrity Figure 5-41 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription • ~ 150 genes control telomere length in yeast and shortening telomeres is associated with cell senescence and death • telomerase highly active in >90% of tumors i.e. cell immortalization and uncontrolled proliferation (drug target) • many adult cell types have detectable telomerase activity, but it is a highly regulated, fine tuned activity MOLECULAR BIOLOGY – DNA replication, transcription Correlation between levels of perceived stress and telomere length and action of telomerase RELAX AND KEEP YOUR TELOMERES LONG! MOLECULAR BIOLOGY – DNA replication, transcription Telomere and Telomerase video/ tutorial http://www.youtube.com/watch?v=AJNoTmWsE0s MOLECULAR BIOLOGY – DNA structure, genetic code TRANSCRIPTION double stranded DNA 5’ 3’ ATG GCT CCT TCT TCC AGA GGT GGC . . . . . . TAA TAC CGA GGA AGA AGG TCT CCA CCG . . . . . . ATT 3’ 5’ TRANSCRIPTION single stranded mRNA TRANSLATION AUG GCU CCU UCU UCC AGA GGU GGC . . . . . . UAA AUG UAA protein coding sequence or open reading frame MAPSSRGG….. Functional Protein Focus on how the genetic information contained within DNA is copied into RNA and it’s consequences MOLECULAR BIOLOGY – DNA replication, transcription Eukaryotic mRNAs are not synthesised in a form that can be immediately translated to protein RNA processing i.e. there are processing and translocation steps Figure 6-21 Molecular Biology of the Cell (© Garland Science 2008) translocation MOLECULAR BIOLOGY – DNA replication, transcription Polycistronic mRNA (e.g related gene operons) Monocistronic mRNA Figure 6-22a Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Table 6-1 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription TRANSCRIPTION INITIATION Single stranded mRNA RNA polymerse complex Doubel stranded DNA How does the RNA polymerase recognise the the correct place within DNA to start transcribing mRNA for a gene? MOLECULAR BIOLOGY – DNA replication, transcription TRANSCRIPTION START IN PROKARYOTES The core ‘RNA polymerase complex’ (consisting of & ’catalytic and 2x regulatory subunits) alone is unable to recognise and bind DNA Molecular interaction with the ‘sigma () factor’ permits RNA polymerase (designated ‘holoenzyme’) to bind DNA at specific regions called ‘promoters’ Figure 6-11 (part 1 of 7) Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription The sigma factor recognises ‘consensus’ sequences in the promoter DNA There are x2 consensus sequences at the -35 and -10 positions of the DNA The sigma factor recognises -35 & -10 and positions the RNA polymerase in the correct position to start synthesis of mRNA starting from the +1 position -35 -10 +1 -35 -10 PROMOTER 1st ribonucleotide to be incorporated into mRNA +1 GENE Variations in the -35 & -10 DNA sequences of different gene promoters affect how often mRNA synthesis can be initiated. Additionally bacteria have multiple sigma factors each with subtle differences in binding affinity for -35 & -10 sequence variants. Figure 6-12a Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription After initiation, sigma factor disassociates and synthesis of the new mRNA molecule occurs in a 5’ - 3’ direction i.e. ‘elongation phase’ 3’ 5’ 5’ The mRNA sequence is specified by base-pair complementarities with the ‘template/ non-coding/ antisense’ DNA strand and therefore is a copy/ ‘transcript’ of the ‘non-template/ coding/ sense’ strand wit U replacing T MOLECULAR BIOLOGY – DNA replication, transcription How does RNA polymerase know when to stop? Just as promoter sequences in DNA instruct where transcription should begin, other DNA sequences called ‘terminators’ specify where it should stop. ‘terminators’ sequences are inverted DNA repeats followed by a run of A nucleotides The repeats are copied into the transcribed mRNA and form a ‘hairpin loop’ that is sensed by the RNA polymerase causing it to pause/ stutter. Weak hydrogen bonding between the run of A nucleotides in the DNA template strand and the U ribonucleotides of the mRNA cause the disassociation of the whole RNA polymerase complex i.e. ‘intrinsic termination’ In other cases the binding of special helicases called ‘Rho ()proteins’ facilitate the disassociation i.e. ‘rhodependent termination’ MOLECULAR BIOLOGY – DNA replication, transcription Prokaryotic transcription cycle video/ tutorial http://highered.mcgraw-hill.com/sites/0072556781/student_view0/chapter12/animation_quiz_1.html MOLECULAR BIOLOGY – DNA replication, transcription TRANSCRIPTION IN EUKARYOTES Additionally transcription occurs in the nucleus and not in the cytoplasm Table 6-2 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Recognition of the promoter and the initiation of transcription in eukaryotes is more complex In addition to RNA polymerase a host of other proteins known as the ‘General Transcription Factors (GTFs)’ are required i.e. PROTEIN CODING GENE TRANSCRIPTION GTF’s help RNA polymerase recognise a T/A rich DNA sequence motif in the promoter called the ‘TATA box’, correctly position it on the ‘chromatin template’ with respect to the +1 position and convert it to form capable of RNA synthesis i.e. help form a ‘pre-initiation complex’ Table 6-3 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Binding of ‘specific transcription factors’ positively or negatively affect frequency of transcription initiation • specific transcription factors bind particular DNA sequences only found in the locality of certain genes • therefore can help regulate which genes mRNAs are synthesised within a cell (e.g. confine the production of antibodies in white blood cells but not in neurones!) e.g. ‘short-range upstream regulator elements’ e.g. ‘long range enhancer sequences’ Specific transcription factor binding Pre-initiation complex of RNA polymerase II and GTFs at TATA box MOLECULAR BIOLOGY – DNA replication, transcription CORE PROMOTER Figure 6-19 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Initiation of eukaryotic transcription video/ tutorial http://bcs.whfreeman.com/thelifewire/content/chp14/1402002.html MOLECULAR BIOLOGY – DNA replication, transcription RNA PROCESSING in Eukaryotes Unlike prokaryotes, the DNA sequence encoding eukaryotic protein is not often organised in one continuous length that when transcribed is a fully functional mRNA that can be translated into protein. The mRNA first needs to be ‘processed’ Eukaryotic genes typically consist of segments of protein coding DNA sequence called ‘exons’ that are interspersed with non-protein coding sequences called ‘introns’ (that are often very long). • both the exons and introns are transcribed by RNA polymerase II to yield long RNA molecules called ‘Pre-mRNAs’ Pre-mRNA CAP AAA(n) SPLICING Mature mRNA CAP AAA(n) • Pre-mRNAs are modified (cotranscriptionally) by addition of a 5’-cap and 3’ polyadenylation motifs (important for stability and translation). • the sequence corresponding to the introns is removed from the pre-mRNA in a process called ‘SPLICING’ to yield a ‘mature mRNA’ • the ‘spliced’ together protein coding mRNA sequence corresponding to the exons in the DNA can now be translated into function protein (except for ‘untranslated regions/ UTRs’ at the 5’ & 3’ ends MOLECULAR BIOLOGY – DNA replication, transcription Capping Eukaryotic Pre-mRNA RNA terminal phosphatse Leads to the covalent attachment of a ‘7-methylguanosine cap (m7G cap)’ at the 5’ end of the premRNA via an unusual 5’ - 5’ triphosphate bond ‘Enzyme capping complex (CEC)’ Bound to RNApolII complex and caps the pre-mRNA cotranscriptionally The m7G cap ensures: • the (pre)mRNA is protected from degradation • mRNA export from nucleus • mRNA is translated to give functional protein Figure 6-22b Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Polyadenylation of Eukaryotic Pre-mRNA Cleavage and polyadenylation specificity factor (CPSF) & cleavage stimulation factor (CstF) PAP extends the 3’ end of the RNA by untemplated addition of up to 200 adenosine (A) nucleotides that are then bound by PABPs CPSF & CstF recognise the cleavage and polyadeylation signals once they have been transcribed into the pre-mRNA CPSF & CstF binding recuits other factors that cleave the pre-mRNA chain free of the RNA polymerase II complex Additionally polymerase (PAP) and poly-A-binding proteins (PABPs) are also recruited to this polyadenylation complex Polyadenylation importance: • participates in transcriptional termination • protects mRNA from degradation • mRNA export from nucleus Figure 6-38 Molecular Biology of the Cell (© Garland Science 2008) • mRNA translation to give functional protein MOLECULAR BIOLOGY – DNA replication, transcription mRNA capping and polyadenylation video/ tutorial http://www.youtube.com/watch?v=YjWuVrzvZYA MOLECULAR BIOLOGY – DNA replication, transcription SPLICING of Eukaryotic Pre-mRNA The very precise removal of non protein coding intron derived sequences from pre-mRNAs (N.B. genetic code) is catalysed by the multiple subunit containing complex called the ‘SPLICEOSOME’ Spliceosome Pre-mRNA The subunit composition of the spliceosome changes as the splicing of introns progresses The main class of subunit are the ‘small nuclear ribonucleoproteins/ snRNPs (“snurps”)’ snRNA snRNPs are complexes of proteins and ‘small nuclear RNA (snRNA)’ The snRNAs in snRNPs permit spliceosome assembly & There are 5 different snRNPs designated U1, U2, U4, U5 & U6 Protein Recoginse ‘specific splice signals’ within the premRNA by complementary base-paring MOLECULAR BIOLOGY – DNA replication, transcription snRNAs of snurps recognize 3 types of splice signal in the pre-mRNA 5’ splice site ‘branch site’ site 3’ splice This adenosine is critical to successful splicing The recognition of these 3 splice signal sequences directs the correct assembly of the spliceosome and the correct joining of the exons Figure 6-28 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Spliceosome reaction mechanism Cowboy’s lariat 2’ - 5’ phosphodiester bond Figure 6-26a Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription Where do the snRNPs fit in? N.B. the dynamics in the composition of the spliceosome between catalysing the first and second phosphoryl-transfer reactions Figure 6-29 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription snRNP & snRNA interactions in the two forms of spliceosome active site Formation of the lariat with the conserved adenosine (A) in the branch site (i.e catalysis of the 2’ -5’ phosphodiester bond) Figure 6-30c Molecular Biology of the Cell (© Garland Science 2008) Excision of the lariat and joining of the two exons (i.e. a conventional 3’ -5’ phosphodiester bond) MOLECULAR BIOLOGY – DNA replication, transcription General splicing video/ tutorial http://bcs.whfreeman.com/thelifewire/content/chp14/1402001.html MOLECULAR BIOLOGY – DNA replication, transcription Self splicing introns Secondary structure formation within the intron caused by complementary base-pairing between its nucleotides forms inherent enzymatic activities (i.e. ‘ribozymes’) that catalyse the removal of the intron and the joining of exons As with spliceosome assisted splicing a conserved adenine (A) nucleotide in the intron participates in the reaction and the intron is excised as a lariat Unicorporated guanine (G) particpates as a cofactor and intron excised as a linear moleclue Some lower eukaryotic species e.g. tetrahymena Some fungi and plants species Figure 6-36 Molecular Biology of the Cell (© Garland Science 2008) MOLECULAR BIOLOGY – DNA replication, transcription ALTERNATIVE SPLICING Alternative splicing is a mechanism by which one gene can code for more than one version of a protein depending upon which exons make it into the mature mRNA and are translated. Therefore provides an evolutionary mechanism for extra diversity! Figure 6-31 Molecular Biology of the Cell (© Garland Science 2008)