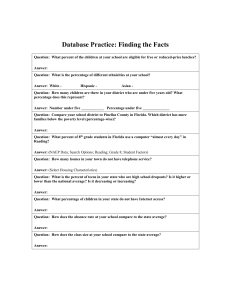
Thermal Cycles - Rankine Cycle with Reheat - plaza
advertisement

Fundamentals Exam UNIVERSITY OF FLORIDA Fundamentals Exam Thermodynamics Review Fundamentals Exam I assume you have applied?!? Morning Session: UNIVERSITY OF FLORIDAGeneral – 120 questions, ½ pt each Afternoon Session: General or Discipline Specific- 60 questions, 1 pt each Fundamentals Exam What to do in the afternoon? Your call...pass/fail is about the same. UNIVERSITY OF FLORIDA Preparation easier for general. Fundamentals Exam UNIVERSITY OF FLORIDA Morning Session: 11 out of 120 thermo questions Afternoon General Session: 6 out of 60 thermo questions Fundamentals Exam UNIVERSITY OF FLORIDA NCEES Reference Handbook Have you got it? Why not? How do you get it? How do you use it? www.ncees.org Fundamentals Exam Morning session Generally unrelated. About 2 minutes UNIVERSITY OF per question. FLORIDA Fast recall essential. Use a marking system to keep track of your progress. Afternoon: 4 minutes per question. Fundamentals Exam Process of Elimination Cross out wrong answers first. UNIVERSITY OF FLORIDA Wrong answers are sometimes easier to find than right ones! Units on answer is sometimes a clue. Answers are seldom given with more than 3 sig figs, your choice should be the closest to your solution. Fundamentals Exam Guessing No penalty for guessing. UNIVERSITY OF FLORIDA Leave 10 minutes for each session for “educated” guessing. Fundamentals Exam Hint: Write correct answer in the margin of UNIVERSITY OF your test booklet beside the question FLORIDA and wait to you get to the end of the page before transferring to the answer key. Lookout for those long, drawn out questions… questions with four paragraphs for answers!! Fundamentals Exam Try working the following problem (you have two minutes for each type problem like this): If a sample experiencing a change of temperature from 23 deg C to 46 deg C also experiences a change in specific UNIVERSITYenthalpy OF of 120 kJ/kg, of what material is the sample most likely to be composed? You can use the data in the NCEES FLORIDA Supplied Reference Handbook or the tables in the back of your book. Better to practice these problems with your NCEES handbook…..remember to be at one with this book!! Did you get Helium? There are sample tests on the NCEES website. Try these out. There are also sample tests in books like Barron’s How to Prepare for the Fundamentals of Engineering FE/EIT Exam. The bookstores have books like this to help you review for the exam. Fundamentals Exam Select the best response for an isolated system. UNIVERSITY OF a. The entropy of the system remains FLORIDA constant b. The heat transfer equals the work done c. The heat transfer equals the internal energy change d. The heat transfer is zero. Fundamentals Exam Select the best response for an isolated system. a. The entropy of the system remains constant b. The heat transfer equals the work done UNIVERSITY OF c. The heat transfer equals the internal energy FLORIDA change d. The heat transfer is zero. For a closed thermodynamic system: Q – w = DU + DKE + DPE, isolated implies Q = W = 0, (d) is the answer, (b) is close but not complete Fundamentals Exam Two kilograms of air are contained in a cylinder. If 80 kJ of heat are added to the air, estimate the temperature rise if UNIVERSITY OF FLORIDA the pressure is held constant. Cp = 1.0 kJ/kgK, Cv = 0.716kJ/kgK and k = 1.4. a. 56 deg C b. 40 deg C c. 33 deg C d. 28 deg C Fundamentals Exam Two kilograms of air are contained in a cylinder. If 80 kJ of heat are added to the air, estimate the temperature rise if the pressure is held UNIVERSITY OF constant. Cp = 1.0 kJ/kgK, Cv = FLORIDA 0.716kJ/kgK and k = 1.4. a. 56 deg C b. 40 deg C c. 33 deg C d. 28 deg C Answer is (b) Q = mDh = m CpDT, 80 = 2*1.0*DT, DT= 40 deg C Fundamentals Exam Steam at high temperature and pressure passes through a half open globe valve. UNIVERSITY OF Select the property that remains FLORIDA constant through the valve. a. enthalpy b. temperature c. pressure d. entropy Fundamentals Exam Steam at high temperature and pressure passes through a half open globe valve. Select the property that remains constant through the valve. UNIVERSITY OF a. enthalpy FLORIDA b. temperature c. pressure d. entropy Answer is (a), energy equation q-ws = Dh + Dpe + Dke, q = 0, ws = 0, Dpe = 0, Dke=0 therefore Dh = 0, an isenthalpic process, enthalpy is constant Fundamentals Exam For an isentropic process of an ideal gas (k= 1.4), with an initial pressure of 50 pounds per square inch absolute, an initial specific UNIVERSITY OF volume of 8.2 cubic feet per pound mass, and FLORIDA a final pressure of 120 psia, what is the final value of the specific volume? a. 8.2 cubic feet/lbm b. 3.42 cubic feet/lbm c. 19.7 cubic feet/lbm d. 4.39 cubic feet/lbm Fundamentals Exam For an isentropic process of an ideal gas (k= 1.4), with an initial pressure of 50 pounds per square inch absolute, an initial specific volume of 8.2 cubic feet per pound mass, and a final pressure of 120 psia, what is the final value of the specific volume? UNIVERSITY OF a. 8.2 cubic feet/lbm FLORIDA b. 3.42 cubic feet/lbm c. 19.7 cubic feet/lbm d. 4.39 cubic feet/lbm Answer is (d). P1v1k = P2v2k, v2 = v1(P1/P2)1/k = (8.2)*(50/120)1/1.4 = 4.39 ft3/lbm Fundamentals Exam How much energy must be transferred through heat interaction to raise the UNIVERSITY OF temperature of a 4 kilogram sample of FLORIDA methane in a closed system from 15 deg C to 35 deg C? a. 34.8 kJ b. 45 kJ c. 139 kJ d. 180 kJ Fundamentals Exam How much energy must be transferred through heat interaction to raise the temperature of a 4 kilogram sample of methane in a closed system from 15 deg C to 35 deg C? UNIVERSITY OF a. 34.8 kJ FLORIDA b. 45 kJ c. 139 kJ d. 180 kJ Answer is ( c). Q- w = DU + DKE + DPE Closed system. Q = DU = mDu = mCvDT = (4kg)*(1.74kJ/kg K)*(35-15 deg C)= 139.2 kJ Fundamentals Exam A tank contains 0.02m3 of liquid and 1.98 m3 of vapor. If the density of the liquid is 960 3 and that of the vapor is 0.5kg/m3, what kg/m UNIVERSITY OF FLORIDA is the quality of the mixture? a. b. c. d. 5.2% 4.9% 2.04% 1.01% Fundamentals Exam A tank contains 0.02m3 of liquid and 1.98 m3 of vapor. If the density of the liquid is 960 kg/m3 and that of the vapor is 0.5kg/m3, what is the quality of the mixture? UNIVERSITY OF a. 5.2% FLORIDA b. 4.9% c. 2.04% d. 1.01% Answer is (b). x = mg/(mg + mf) = (1.98)*(.5)/((1.98*0.5)+(0.02*960)) = 0.049 or 4.9% Fundamentals Exam Which of the following is an intensive property? UNIVERSITYa. OF Pressure FLORIDA b. Entropy c. Internal Energy d. Enthalpy Answer is (a) Pressure does not depend on mass , extensive properties are proportional to mass Fundamentals Exam Which of the following devices is possible? a. A cyclic machine that will experience no other interaction than to produce energy through a work interaction, while transferring energy from a high-temperature reservoir to a low-temperature reservoir through heat interactions. b. A cyclic machine that will experience no other interaction than to transfer to a thermal reservoir an amount of energy equal to the amount of energy it UNIVERSITY OF receives from a work interaction. c. A device that will change the thermodynamic state of a material from on FLORIDA equilibrium state to another without experiencing a change in the amount of energy contained in the material, in the amount of material, or in the external forces placed on the material. d. A cyclic machine that will experience no other interaction than to accept from a heat interaction with a high-temperature reservoir an amount of energy equal to the amount of energy it receives from a work interaction. Answer is (a). Note that this problem takes about a minute to read!! You better understand this one as you read it or you won’t do this in 2 minutes!! (b) is not right because entropy decreases continuously, (c) is not right because you can’t be in two equilibrium states, (d) is not correct because energy is increasing continuously Fundamentals Exam Energy is added in the amount of 50 kJ in a heat interaction to a closed system while 30 kJ of work is done by the system. The change of the internal energy of the system is: UNIVERSITYa. OF 80 kJ FLORIDA b. 20 kJ c. –20kJ d. –80kJ Answer is (b). DE = Q-W = +50kJ-(+30kJ) = 20 kJ This is an example of how you need to know the first law and know the correct sign conventions for work and energy. Q into a system is + W done by a system is + (remember it is a positive thing to get work out of a student!!) Fundamentals Exam Devices: Turbines UNIVERSITY OF Compressors FLORIDA Diffusers Nozzles Throttling Devices Heat Exchangers Fundamentals Exam Devices: Turbines UNIVERSITY OF Compressors FLORIDA mi’(hi +Vi2/2 + gzi) –me’ (he +Ve2/2 + gze)+Q’ –W’ = 0 Assume well insulated, assume Vi=Ve and steady flow mi’ = me’,and zi = ze Then: m’(hi – he) = W’ Compressors: W’ is – Turbines: W’ is + Fundamentals Exam Devices: Nozzles and Diffusers UNIVERSITY OF 2/2 + gz ) –m ’ (h +V 2/2 + m ’(h +V FLORIDA i i i i e e e gze)+Q’ –W’ = 0 Assume well insulated, no shaft work, and steady flow mi’ = me’,and zi = ze Then: (hi – he +Vi2/2 -Ve2/2 ) = 0 Ve > Vi for nozzles and Ve<Vi for diffusers Fundamentals Exam Devices: Throttling Device UNIVERSITY OF 2/2 + gz ) –m ’ (h +V 2/2 + m ’(h +V FLORIDA i i i i e e e gze)+Q’ –W’ = 0 Assume well insulated, assume Vi=Ve and steady flow mi’ = me’, zi = ze and no shaft work Then: (hi – he) = 0 isenthalpic Fundamentals Exam Devices: Heat Exchangers UNIVERSITY OF 2/2 + gz ) –m ’ (h +V 2/2 + m ’(h +V FLORIDA i i i i e e e gze)+Q’ –W’ = 0 Assume well insulated, assume Vi=Ve and steady flow mi’ = me’, no shaft work and zi = ze Then: m’(hi – he) = Q’ Fundamentals Exam Cycles: Carnot Reversed Carnot UNIVERSITY OF Otto FLORIDA Rankine Refrigeration Afternoon: if mechanical: two stage refrig cycle, air refrigeration, psychrometric cycles, Brayton cycle, Brayton cycle with regeneration, etc. Fundamentals Exam Review state functions, specifically the concept of quality, x UNIVERSITY OF u = xug + (1-x)uf FLORIDA h = xhg + (1-x)hf s = xsg + (1-x)sf v = xvg +(1-x)vf Look at Rankine cycle, steam quality out of a turbine Thermodynamics Review A Carnot engine operates between 300°C and 40 °C. What is the efficiency of the engine? UNIVERSITY OF FLORIDA (A) (B) (C) (D) 87% 65% 45% 30% Thermodynamics Review Solution: (C) See Page 49 in Reference Handbook Carnot Cycle efficiency: FLORIDA UNIVERSITY OF c = (TH TL ) / TH = 1 TL / TH ( 40 273) K c = 1 = .45 = 45% (300 273) K Not (A): If you got (A) you didn’t use the absolute temperature units. 40o C c = 1 = .8666 = 86.7% 45% o 300 C “When in doubt use absolute.” Thermodynamics Review Refrigerant 134a is isentropically compressed in a compressor from a saturated vapor state at 0.4 MPa UNIVERSITY OF pressure to 2 MPa pressure. The work required to run FLORIDA the compressor is: (A) 130 kJ/kg (B) -100 kJ/kg (C) 100kJ/kg (D) -60kJ/kg Thermodynamics Review Solution: (B) See Pages 48 and 55 in Reference Handbook for First Law (energy OF balance) and p-h table for Refrigerant 134a UNIVERSITY FLORIDA Use the Chart: The question states that the refrigerant is in a saturated vapor state, therefore the enthalpy can be obtained by finding the intersection line for the given pressures and the right side of the “dome” respectively. hi 330(kJ / kg ) and he 430(kJ / kg) The exit enthalpy has the same s as the inlet but at a higher P. Energy balance: W = hi he = 330 (kJ / kg ) 430( kJ / kg ) = 100 (kJ / kg ) The answer is negative because work is put into the system. Thermodynamics The Air Standard Assumptions • for gas powered cycles UNIVERSITY OF FLORIDA • for Otto cycle, diesel cycle and Brayton cycle • the working fluid is air which continuously circulates through the system, acts as an ideal gas • all processes are internally reversible • combustion process is approximated by a heat addition process from external source • exhaust process is approximated by a heat rejection process Thermodynamics The Cold Air Standard Assumptions • all of above assumptions UNIVERSITY OF FLORIDA • assumes specific heats are constant and are evaluated at 25 deg C or 77 deg F • all of the above assumptions are made to simplify a very complex cycle Thermodynamics The Otto Cycle, aka SIE, Spark Ignition Engine • This cycle applies to two stroke and four stoke FLORIDA engines. It is an ideal representation of the process. UNIVERSITY OF 1-2: isentropic compression (compression stroke) 2-3: constant volume heat addition (power stroke) 3-4: isentropic expansion (exhaust stroke) 4-1: constant volume heat rejection (intake stroke) Thermodynamics UNIVERSITY OF FLORIDA Thermodynamics Model this as a closed system. No major changes in kinetic or potential energy. Energy equation becomes: UNIVERSITY (q -q )+(wOF-w in out in FLORIDA out) = Du But more importantly, let’s look at the processes from 2 to 3 and 4 to 1: qin = u3-u2 = Cv(T3-T2) qout = u4 – u1 = Cv(T4 – T1) (no work done during these processes) th,Otto = wnet/qin Looking at the first law, in = out wnet = qin – qout Therefore, th,Otto = wnet/qin = (qin-qout)/qout Thermodynamics th,Otto = wnet/qin = (qin-qout)/qout Substituting for qin and qout th,Otto = 1 – ((T4-T1)/(T3-T2) UNIVERSITY OF FLORIDA Since 1 to2 and 3 to 4 are isentropic processes, we can substitute our isentropic relationships for T and v. Therefore, (see derivation on page 360) thOtto = 1 1 r k 1 Where the compression ratio r = V1/V2 = v1/v2 And k = specific heat ratio = Cp/Cv Thermodynamics Let’s look at an example: Assume a compression ratio of 8. Assume airOF at the beginning of the process UNIVERSITY is at 17 deg C and 100KPa. FLORIDA Assume 800 kJ/kg of heat is added in the heat addition process (this would be equivalent to the fuel added to the cycle). Assume constant specific heats. The efficiency of this cycle would equal: thOtto = 1 1 r k 1 = 1 1 8 (1.4 1) = 0.565or 56.5% Thermodynamics Let’s find the temperature at 3. This would be the maximum temperature our equipment would have to tolerate. qin= 800kJ/kg = Cv(T3-T2)=0.718*(T3 – T2) UNIVERSITY OF We need T2 to solve for T3. FLORIDA From the isentropic relationships, T1/T2 = (V2/v1)k-1 Note that r = v1/v2, Therefore, T1/T2 = (1/r)k-1 And T2 = 290K/(1/8) (1.4-1) T2 = 666 K So T3 = (800/0.718)+666 = 1780 K or 2745deg F Can also do this assuming variable specific heats. Thermodynamics Let’s find the pressure at 3. This would be the maximum pressure our equipment would have to tolerate. P1v1/T1 = P2v2/T2 UNIVERSITY OF Therefore P2 = P1v1T2/v2 T1 FLORIDA P2 = 100kPa(666K/290K)(8) = 1837 kPa And P2v2/T2 = P3v3/T3 P3 = P2T3v2/T2v3 P3 = (1837kPa)(1780K/666K)(1) Note v2 = v3 P3 = 4910 kPa or 712 psia!! Thermodynamics The Brayton Cycle http://travel.howstuffworks.com/turbine3.htm • Used to analyze gas turbines (where compression and expansion occur using rotating machinery) UNIVERSITY OF FLORIDA • Gas Turbines usually operate as an open cycle • Used in aircraft propulsion and electric power generation Exhaust propels craft or used to generate steam. Thermodynamics Simple Ideal Brayton Cycle UNIVERSITY OF FLORIDA Modeled as a closed cycle. Air standard assumptions are applied. Air is the working fluid. 1-2: isentropic compression 2-3: constant pressure heat addition 3-4: isentropic expansion 4-1: constant pressure heat rejection Thermodynamics Model this as a closed system. No major changes in kinetic or potential energy. qin = h3 – h2 = Cp (T3 – T2) -q out = h1 – h 4 = Cp(T1 – T4) UNIVERSITY OF FLORIDA Or qout = Cp(T4 – T1) th,Brayton = wnet/qin Looking at the first law, in = out wnet = qin – qout Therefore, th,Brayton = wnet/qin = (qin-qout)/qin th,Brayton = 1 – T1(T4/T1-1)/T2(T3/T2-1) Substituting the isentropic relationships for T as a function of P and realizing that P2=P3 and P1 = P4, th,Brayton = 1 – 1/rp (k-1)/k where rp = pressure ratio = P2/P1 Thermodynamics • For gas turbine engines, the rp ranges from 5 t0 20. • Since some of the turbine work goes to run the compressor, there is another term used to describe this cycle: UNIVERSITY OF the back work ratio = Compressor work/turbine work. FLORIDA • Usually more than half the turbine work goes to run the compressor. • The back work ratio for steam power plants is very low in comparison. • Gas turbines used in power plants can be brought on line very quickly whereas the Rankine cycle steam cycles take a lot of time to bring up to speed. This is why gas turbine engines are used as “peaking” units. • With improvements in firing temperature, turbomachinery efficiency and heat recovery, the gas turbine power generating systems are now comparable to steam plants in performance, especially when the waste heat is combined with a Rankine cycle plant (bottoming cycle). Thermodynamics Let’s look at an example: Assume a pressure ratio of 8. Assume theOFair at the compressor inlet (pt UNIVERSITY 1)is at 300K (room temperature). FLORIDA Assume the air at the turbine inlet(pt 3) is at 1300 K. (1880 deg F) Find the gas temperatures at the exits of the turbine and compressor. Find the back work ratio. Find the thermal efficiency. Thermodynamics Using the cold air standard assumptions and assuming negligible changes in kinetic and potential energy: R air = 0.3704 psia ft3/lbm R UNIVERSITY OF CFLORIDA p = 0.24 Btu/lbm R K = 1.4 th,Brayton = 1 – 1/r (k-1)/k th,Brayton = 1 – 1/(8) (1.4-1)/1.4 th,Brayton = 0.448 or 44.8% T2/T1 = (8) (1.4-1)/1.4= 1.811 T2 = (300)( 1.811 ) = 543.4K And T4/T3 = (1/8) (1.4-1)/1.4 = .552 T4 = (1300)(0.522) = 717.6K Thermodynamics qin = h3 – h2 = Cp (T3 – T2) qin = ( 1.005 kJ/kg K)(1300-543.4) UNIVERSITY OF FLORIDA q = C (T out p 4 – T1) = (1.005 kJ/kg K)(717.6-300) th,Brayton = (qin-qout)/qin = 0.448 or 44.8% Back work ratio = Cp(T2-T1)/Cp(T3-T4) = (543.5-300)/(1300-717.6) = 0.42 Thermodynamics Using air standard assumptions (see text): Back work ratio = 0.402 Thermal efficiency = 42.6% UNIVERSITY OF TFLORIDA 2 = 540K T4 = 770 K Our cold air standard assumptions worked well. Thermodynamics The Rankine or Vapor Power Cycle • Used to steam power plant operations UNIVERSITY OF FLORIDA Thermodynamics Simple Ideal Rankine Cycle 1-2 isentropic compression with a pump 3-4 constant pressure heat addition in a boiler UNIVERSITY OF 5-6FLORIDA isentropic expansion in a turbine 6-1 constant pressure heat rejection in condenser Other points can be used to describe pipe Losses (thermal and pressure) Thermodynamics We will consider water(steam) as our motive fluid. The steam leaving the boiler (pts. 4 and 5) is usually superheated steam. The steam leaving the turbine (pt 6) is UNIVERSITY OF FLORIDA usually high quality steam. Remember that s5 = s6 The water leaving the condenser is either saturated liquid or subcooled liquid water. We usually assume that h2 = h1. Or we can estimate h2 = h1 + v(P2-P1). Let’s work an example problem to illustrate how this works. Thermodynamics Consider a steam power plant and assume an ideal Rankine cycle to model the system. P The steam enters the turbine at 3MPa and 350 C UNIVERSITY OF Condenser pressure is at 75 kPa. 4 1 FLORIDA What is the thermal efficiency for this cycle? th,Rankine = wnet/qin= (qin – qout)/qin qin = h1 – h4 3 2 qout = h3-h2 h1 = h of superheated steam = 3115.3 kJ/kg s1 = s2 = 6.7428 kJ/kg K s2 = sf75kPa + x sfg75kPa = 6.7428 kJ/kg K x = (6.7428-1.213)/6.2434 = 0.88 Using x and hf75kPa and hfg75kPa, we can calc. h2 = 384.39+(0.88)(2278.6)= 2402.6 kJ/kg h Thermodynamics h3 = hf75kPa = 384.39 kJ/kg h4 = h3 approx. qin = h1 – h4= 3115.3 – 384.39 kJ/kg = 2730.9 kJ/kg UNIVERSITY OF P 4 1 qout = h3-h2 = 384.39 - 2402.6 = -2018.2 kJ/kg FLORIDA th,Rankine = (2730.9-2018.2)/2730.9 = 26% Note that I calculated the same efficiency neglecting any change in h across the pump. I assumed the pump was isenthalpic instead of isentropic. If we do consider the pump Dh = 0, then the pump work is approx. zero and the back work ratio = 0. If the work of the pump is calculated we find that the back work ratio is 0.004 or 0.4%. Compare to the 0.42 for the Brayton cycle. 3 2 h