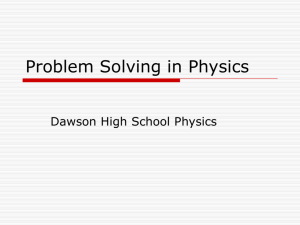
2 x 10 -3
advertisement

Working with Numbers Metric Conversions Following is a list of the metric conversions most frequently used in chemistry. Commit these to memory: Mass: 1000 g = 1 kg 1000 mg = 1 g 1 x 106 µg = 1 g Length: 1000m = 1 km 100 cm = 1 m 1000 mm = 1 m 1 x 109nm = 1 m 1 x 1012 pm = 1 m 1x1010Å = 1m Volume: 1mL = 1 cm3 1000 mL = 1 L English to metric conversions: (No need to memorize these) 1 inch = 2.54 cm = 0.0254m = 25.4 mm = 2.54 x 107 nm 1 foot = 12 in = 30.48 cm = .3048 m = 304.8 mm 1 liter = 1000mL= 1 x 10-3 m3 = 1.06 qt. 1 mile = 5280 ft = 1.609 x 103 m = .1.609 km 1 pound = .4536 kg = 453.6 g = 16 oz. 1 metric ton = 1000kg 1 calorie = 4.184 Joules Significant Figures: See rules on pages 46-50 in textbook. 1. 41.8 has __3__ sig figs 3. 300.7 has __4__ sig figs 2. 0.017 has __2__ sig figs 4. 0.00620 has __3__ sig figs 5. 25.37 + 5.198 + 147.2 = 177.8 6. 7.198 x 3.1414 = 22.61 7. (2.50)4 = 39.1 Dimensional Analysis Using unit conversions to solve problems (Also called factor label, and unit analysis) Example 1: How many Ibs are equal to 32.2 kg? 32.2kg 1 lb .4536kg 71.0 lb Example 2: How many square feet are contained in a square mile? 1 mi2 (5280ft)2 (1mi)2 2.78784 ft2 Example 3: If my car gets 28.0 miles per gallon, how many km per liter does it get? 28.0 mi gal 1 gal 4 qt. 1.06 qt. L 1.609 km 1 mi 11.9 km L Practice Problems Solve the following problems using dimensional analysis. For full homework credit you must use the grid, show all units, and use the correct number of sig figs. 1. 4.63 qt = ____4.37____ L 2. 12.3 ft = _____3750___ mm 3. 5.35 Ib = ___2.43 x 106___ mg 4. 6 yd2 = _____5______ m2 5. 50cm3 = _____2 x 10-3 __ ft3 6. 12 cubic yards = __9.2 x 103__ L 7. 156 mL = ___0.165___ qt. 8. 7.2 oz./gal = ____54_____ mg/mL 9. 354 g/L = ____2.94 ____ Ib / gal 10. 55 mi/hr = ____25______ m/s Chemistry I Worksheet Density and Specific Gravity 𝑫𝒆𝒏𝒔𝒊𝒕𝒚 𝒐𝒇 𝒂 𝒔𝒖𝒃𝒔𝒕𝒂𝒏𝒄𝒆 = 𝑴𝒂𝒔𝒔 𝒐𝒇 𝑺𝒖𝒃𝒔𝒕𝒂𝒏𝒄𝒆(𝒈) 𝑽𝒐𝒍𝒖𝒎𝒆 𝒐𝒇 𝑺𝒖𝒃𝒔𝒕𝒂𝒏𝒄𝒆 (𝒎𝑳) Solve the following problems: Show and cancel all units, use the correct number of significant digits, and use scientific notation when necessary. 1. Calculate the density in g/mL for a piece of metal of volume 60 cm3 and mass 3.00 x 102 grams. (ans 5 g/mL) 2. Calculate the volume in Liters of a sample of a liquid having a mass of 65.0 g and a density of 1.60 g/mL. (ans 0.0406 L) 3. Calculate the volume in milliliters of a solution having a mass of 0.185 kg, and a density of 1.26 g/mL. (ans 147 mL) 𝑺𝒑𝒆𝒄𝒊𝒇𝒊𝒄 𝑮𝒓𝒂𝒗𝒊𝒕𝒚 = 𝑫𝒆𝒏𝒔𝒊𝒕𝒚 𝒐𝒇 𝒂 𝒔𝒖𝒃𝒔𝒕𝒂𝒏𝒄𝒆 (𝒈/𝒎𝑳) 𝑫𝒆𝒏𝒔𝒊𝒕𝒚 𝒐𝒇 𝑾𝒂𝒕𝒆𝒓 (𝟏 𝒈/𝒎𝑳) Since the density of water is 1 g/ml, Specific Gravity and density are numerically equal. The units of density are: _g/mL or kg/L__ The units of Specific Gravity are: __None_______ 1. Calculate the volume in mL occupied by a sample of sulfuric acid having a mass of 285 grams, and a specific gravity of 1.83. (ans 156mL) 2. A sample of acetic acid has a mass of 0.725 kg, and a specific gravity of 1.05. What is its volume in Liters? (ans 0.690 L) 3. What is the mass in grams of a 20.0 mL volume of benzene, if benzene has a specific gravity of 0.880? (ans 17.6 g) 4. Calculate the mass in grams of 150 mL of acetic acid. (Specific gravity 1.05) (ans 160 g)