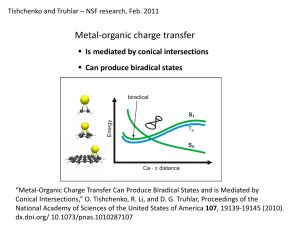
The Role of Conical Intersections in Non
advertisement

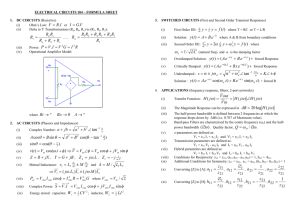
Conical Intersections Spiridoula Matsika The Born-Oppenheimer approximation Energy TS Nuclear coordinate R The study of chemical systems is based on the separation of nuclear and electronic motion The potential energy surfaces (PES) are generated by the solution of the electronic part of the Schrodinger equation. This solution gives an energy for every fixed position of the nuclei. When the energy is plotted as a function of geometries it generates the PES as a(3N-6) dimensional surface. Every electronic state has its own PES. On this potential energy surface, we can treat the motion of the nuclei classically or quantum mechanically Hamiltonian for molecules The total Hamiltonian operator for a molecular system is the sum of the kinetic energy operators (T) and potential energy operators (V) of all particles (nuclei and electrons). In atomic units the Hamiltonian is: H T V H tot (r,R) T N T e V ee V eN V NN Z Z 1 2 1 2 1 Z i 2M i 2m e i ji rij i ri R T N H e (r;R) 1 2 H T H H e (r;R) 2M T N e Assuming that the motion of electrons and nuclei is separable, the Schrodinger equation is separated into an electronic and nuclear part. R and r are nuclear and electronic coordinates respectively. The total wavefunction T is a product of electronic Ie and nuclear I wavefunctions for an I state. T (r,R) I (R)Ie (r;R) H T T E T T H E e e I e I e I Electronic eq. (T N E Ie ) I E T I Nuclear eq. Nonadiabatic processes are facilitated by the close proximity of potential energy surfaces. When the potential energy surfaces approach each other the BO approximation breaks down. The rate for nonadiabatic transitions depends on the energy gap. Energy Avoided crossing Nuclear coordinate R When electronic states approach each other, more than one of them should be included in the expansion Na T (r,R) I (R)Ie (r;R) Born-Huang expansion I 1 If the expansion is not truncated the wavefunction is exact since the set Ieeis ecomplete. e e The total Schrodinger equation using the Born-Huang H I E Ibecomes I expansion (T N 1 K II E Ie ) I N 1 (2f IJ J K IJ J ) E T I J I 2 fIJ (R) eI eJ k IJ (R) eI 2 eJ r r Derivative coupling: couples the different electronic states Derivative coupling fIJ I J I H J EJ EI fIJ fJI fII 0 For real wavefunctions I 2 J fIJ fIJ fIJ The derivative coupling is inversely proportional to the energy difference of the two electronic states. Thus the smaller the difference, the larger the coupling. If E=0 f is infinity. What is a conical intersection Two adiabatic potential energy surfaces cross. The interstate coupling is large facilitating fast radiationless transitions between the surfaces The Noncrossing Rule The adiabatic eigenfunctions are expanded in terms of i 1 c111 c 21 2 2 c121 c 22 2 The electronic Hamiltonian is built and diagonalized H11 H12 H H 21 H 22 e H ij i H e j H H11 H 22 H11 H 22 H 2 H122 E1,2 2 The eigenvalues and eigenfunctions are: 1 cos 1 sin 2 2 2 sin sin cos 2 2 2 2 1 cos 2 2 H12 H 2 H122 H11 H 22 H 2 H122 In order for the eigenvalues to become degenerate: H11(R)=H22 (R) H12 (R) =0 Since two conditions are needed for the existence of a conical intersection the dimensionality is Nint-2, where Nint is the number of internal coordinates For diatomic molecules there is only one internal coordinate and so states of the same symmetry cannot cross (noncrossing rule). But polyatomic molecules have more internal coordinates and states of the same symmetry can cross. J. von Neumann and E. Wigner, Phys.Z 30,467 (1929) Conical intersections and symmetry H11 H12 H H H 21 22 e Symmetry required conical intersections, Jahn-Teller effect • • • Symmetry allowed conical intersections (between states of different symmetry) • • • H12=0, H11=H22 by symmetry seam has dimension N of high symmetry Example: E state in H3 in D3h symmetry H12=0 by symmetry Seam has dimension N-1 Example: A1-B2 degeneracy in C2v symmetry in H2+OH Accidental same-symmetry conical intersections • Seam has dimension N-2 Example: X3 system branching coordinates R Qx Qy r Seam coordinate Qs Figure 4a energy (a.u.) Two internal coordinates lift the degeneracy linearly: g-h or branching plane E 0.015 0.01 0.005 0 h -0.005 -0.01 -0.015 g -0.2 -0.1 y (bohr) 0 0.1 0.2 -0.2 0.1 0 x (bohr) -0.1 Figure 1b E (eV) Nint-2 coordinates form the seam: points of conical intersections are connected continuously 3 2 1 0 -1 -2 -3 0.6 0.4 2.9 0.2 3 0 3.1 r (a.u.) -0.2 3.2 3.3 -0.4 3.4 -0.6 x (a.u.) The Branching Plane The Hamiltonian matrix elements are expanded in a Taylor series expansion around the conical intersection H(R) H(R 0 ) H(R 0 ) R H(R) 0 H(R 0 ) R H12 (R) 0 H12 (R 0 ) R Then the conditions for degeneracy are H(R0 ) R 0 H12 (R0 ) R 0 g H h H12 gx hy H (sx x sy y)I hy gx e E1,2 sx x sy y (gx) 2 (hy) 2 Topography of a conical intersection asymmetry tilt E E0 sxx sy y g 2x 2 h2y 2 Conical intersections are described in terms of the characteristic parameters g,h,s Geometric phase effect (Berry phase) If the angle changes from to +2: 1 cos 1 sin 2 2 2 2 sin 1 cos 2 2 2 1 ( 2 ) 1( ) 2 ( 2 ) 2 ( ) The electronic wavefunction is doubled valued, so a phase has to be added so that the total wavefunction is single valued T e iA(R ) (R;r) (R) The geometric phase effect can be used for the identification of conical intersections. If the line integral of the derivative coupling around a loop is equal to Adiabatic and Diabatic represenation Adiabatic representation uses the eigenfunctions of the electronic hamiltonian. The derivative coupling then is present in the total Schrodinger equation Diabatic representation is a transformation from the adiabatic which makes the derivative coupling vanish. Off diagonal matrix elements appear. Better for dynamics since matrix elements are scalar but the derivative coupling is a vector. Strickly diabatic bases don’t exist. Only quasidiabatic where f is very small. Practically g and h are taken from ab initio wavefunctions expanded in a CSF basis Ie N CSF I c mm m1 H (R) E (R)c (R) 0 e I I Tuning, coupling vectors H(R) J h (R) c (R x ) c (R x ) R IJ I † H(R) I g (R) c (R x ) c (R x ) R I I † gIJ(R)= gI(R) - gJ(R) Locating the minimum energy point on the seam of conical intersections Projected gradient technique: M. J. Baerpack, M. Robe and H.B. Schlegel Chem. Phys. Lett. 223, 269, (1994) Lagrange multiplier technique: M. R. Manaa and D. R. Yarkony, J. Chem. Phys., 99, 5251, (1993) Locate conical intersections using lagrange multipliers: ji Eij g R 0 h R 0 ji Additional geometrical constrains, Ki, , can be imposed. These conditions can be imposed by finding an extremum of the Lagrangian. L (R, , )= Ek + 1Eij+ 2Hij + iKi g 6 h 6 O 5 O 5 4 4 3 H H 3 Y(a0) 0 Branching vectors for OH+OH 2 1 2 1 O O 0 0 -1 H -1 -2 H -2 -4 -3 -2 -1 0 X(a0) 1 2 3 -4 4 -3 -2 -1 0 X(a0) 1 2 3 4 Routing effect: E OH(A)+OH(X) Figure 4a energy (a.u.) Quenching to OH(X)+OH(X) 0.015 0.01 0.005 0 g -0.005 -0.01 h -0.015 -0.2 -0.1 y (bohr) 0 0.1 0.2 -0.2 Reaction to H2O+O -0.1 0.1 0 x (bohr) 0.2 Three-state conical intersections Three state conical intersections can exist between three states of the same symmetry in a system with Nint degress of freedom in a subspace of dimension Nint-5 H11 H H12 H13 H12 H 22 H 23 H13 H 23 H 33 H11(R)=H22 (R)= H33 H12 (R) = H13 (R) = H23 (R) =0 Dimensionality: Nint-5, where Nint is the number of internal coordinates J. von Neumann and E. Wigner, Phys.Z 30,467 (1929) Conditions for a conical intersection including the spin-orbit interaction 1 2 H 11 H *12 H12 H 22 T1 0 H 1T 2 H 11 H12 T2 H1T2 0 * H 12 H 22 In general 5 conditions need to be satisfied. H11=H22 Re(H12)=0 Im(H12)=0 Re(H1T2)=0, satisfied in Cs symmetry Im(H1T2)=0, satisfied in Cs symmetry The dimension of the seam is Nint-5 or Nint-3 C.A.Mead J.Chem.Phys., 70, 2276, (1979)