Nasal Drug Delivery in EMS - Oregon Emergency Medical Services
advertisement

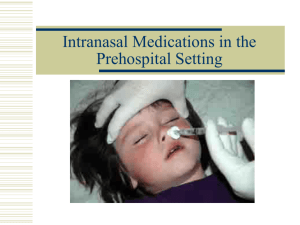
Intranasal Medications in the Prehospital Setting The problem! The CDC estimates: 600,000 percutaneous injuries each year involving contaminated sharps. Technological developments can increase protection. Not being universally used (cost $, cumbersome, time-consuming). …in the field! High risk patients HIV+ patients = 4.1-8.3/100 transports Marcus et al, Ann Em Med, 1995 High risk environments Altered patients, combative Scene control issues Moving ambulance Risk of exposure… Marcus et al, Ann Emerg Med, 1995 Number of exposures in the field: Blood contacts = 4.9/100 shifts Percutaneous = 0.8/100 shifts Reed et al, JEM, 1993 Total exposures = 4.4/1000 EMS calls Needle sticks = 0.24/1000 EMS calls Level III exposures = 1.3/1000 calls ALS personnel = 1.1/1000 ALS calls BLS personnel = 1.4/1000 BLS calls OSHA Occupational Exposure and Bloodborne Pathogens Standard Published in 1991 “…to reduce or eliminate the hazards of occupational exposure, an employer must implement an exposure control plan… The plan must describe how an employer will use a combination of engineering and work practice controls… Engineering controls are the primary means…and include the use of safer medical devices…” OSHA Needle stick Safety and Prevention Act Continued concern over exposures and lack of universal technological developments Signed into Law Nov. 6, 2000 Revised on April 18,2001 “Minimal” requirements for all states Applies to all employers with employees at risk Greater detail on “engineering controls,” such as safer medical devices OSHA Needle stick Safety and Prevention Act. “Non-managerial employees responsible for direct patient care must have input into employer decisions about WHICH engineering controls to adopt” regarding reduction of needle sticks, “not WHETHER or not to adopt them.” Exposure Control Plan The employer must: Take into account innovations in medical procedure and technological developments that reduce the risk of exposure… Document consideration and use of appropriate, commercially-available, and effective safer devices… No one device is considered appropriate or effective for all circumstances Employee input Employers must solicit input Non-managerial employees Direct patient care Identification, evaluation and selection of effective engineering controls, including safer medical devices Employee input must be documented! Safer Medical Devices Needleless devices Needleless hubs Jet injection systems “Needleless” drug administration routes: Nasal, Buccal, Oral, Rectal, Transdermal, etc Shielded needle systems Plastic capillary tubing Intranasal Medication Administration Intranasal Medication administration offers a truly “Needleless” solution to drug delivery. The remainder of this slide show will surround the topic of intranasal drug delivery issues. Intranasal Medication Administration: Basic Concepts This delivery route has several advantages: Its easy and convenient Almost everyone has a nose The nose is a very easy access point for medication delivery No special training is required to deliver the medication No shots are needed It is painless It eliminates any risk of a needle stick to you, the medical provider Understanding IN delivery: Definitions Bioavailability First pass metabolism Nose brain pathway Lipophilicity Bioavailability How much of the administered medication actually ends up in the blood stream. Examples: IV medications are 100% bioavailable. Most oral medications are about 5%-10% bioavailable due to destruction in the gut and liver. Nasal medications discussed in this lecture range in the 55% to 100% bioavailability range - approaching IV delivery systems. First pass metabolism Molecules absorbed through the gut, including all oral medications enter the “portal circulation” and are transported to the liver. Liver enzymes then break down most of these drug molecules and only a small fraction enter the bodies circulation as active drug. This process is called “First Pass Metabolism.” POINT: Nasally delivered medications avoid the gut so do not suffer first pass metabolism. Nose brain pathway The olfactory mucosa (smelling area in nose) is in direct contact with the brain and CSF. Medications absorbed across the olfactory mucosa directly enter the brain. This area is termed the nose brain pathway and offers a rapid, direct route for drug delivery to the brain. Lipophilicity “Lipid Loving.” Cellular membranes are composed on layers of lipid material. Drugs that are lipophilic are easily and rapidly absorbed across the mucous membranes. Intranasal Medication Administration: Advantages Drugs absorbed via the nasal mucosa: Are absorbed via the rich vascular plexus of the nose and directly enter the circulation. Avoid the stomach and small intestine so are not destroyed by acid and digestive enzymes, nor delayed in their absorption to the blood stream. Avoid the portal circulation, so are not subject to extensive destruction by the liver. Can be absorbed directly through the olfactory mucosa into the CSF - giving rapid brain levels of the drug. Intranasal Medication Administration: Advantages Compared to oral medications, intranasal medication delivery results in: Faster delivery to the blood stream Higher blood levels No destruction by stomach acid and intestinal enzymes No destruction by hepatic first pass metabolism Intranasal Medication Administration: Advantages Compared to IV medications, intranasal medication delivery results in: Comparable blood levels depending on the drug and dose. Higher brain levels if well absorbed across the olfactory mucosa. Intranasal Medication Administration: Bioavailability Not all drugs can be delivered via the nasal mucosa. Factors affecting bioavailability: Medication characteristics. Medication volume and concentration. Nasal mucosal characteristics. Delivery system characteristics. Mucosal surface area coverage. Medication particle size. Intranasal Medication Administration: Factors Affecting Bioavailability Medication Characteristics: Drug characteristics that affect bioavailability via the nasal mucosa include: Molecular size. Lipophilicity. pH. Drug concentration. Properties of the solution the drug is solubilized within. Intranasal Medication Administration: Factors Affecting Bioavailability Volume and concentration: Low volume. High concentration. Too large a volume or too weak a concentration may lead to failure because the drug cannot be absorbed in high enough quantity to be effective. Volumes over 1 ml per nostril are likely too dilute and may result in runoff out of the nostril. Intranasal Medication Administration: Factors Affecting Bioavailability Nasal mucosal characteristics: If there is something wrong with the nasal mucosa it may not absorb medications effectively. Examples: Vasoconstrictors s/a cocaine prevent absorption. Bloody nose, nasal congestion, mucous discharge all prevent mucosal contact of drug. Destruction of nasal mucosa from surgery or cocaine abuse – no mucosa to absorb the drug. Intranasal Medication Administration: Factors Affecting Bioavailability Delivery system characteristics: Nasal mucosal surface area coverage: Larger surface area delivery = higher bioavailability. Particle size: Particle size 10-50 microns adheres best to the nasal mucosa. Smaller particles pass on to the lungs, larger particles form droplets and run-out of the nose. Intranasal Medication Administration: Factors Affecting Bioavailability Delivery system characteristics (continued): Atomization results in higher bioavailability than either spray or drops. For this reason, nasal pharmaceuticals come with atomized drug delivery systems. Mucosal Atomization Device (MAD) MAD - Mucosal Atomization device: Device designed to allow emergency personnel to delivery nasal medications as an atomized spray. Broad 30-micron spray ensure excellent mucosal coverage. Intranasal Medication Administration: Factors Affecting Bioavailability Points: Nasal drug delivery is convenient and easy, but it may not always be effective. Nasal drug delivery cannot completely replace the need for injections. Being aware of the limitations and using the correct equipment and drug concentrations will assist you in predicting times when nasal drug delivery may not be effective. Nasal Drug Delivery: What Medications? FDA approved: A large number of medications ranging from nasal steroids to antibiotics to opiate anesthetics are FDA approved. Non-FDA approved: Many other medications are effective via the nasal mucosa but for a number of financial reasons (as opposed to medical reasons) the pharmaceutical companies have not pursued FDA clearance for nasal delivery. Nasal Drug Delivery in EMS: What Medications? Drugs of interest to EMS systems: Intranasal naloxone (Narcan) Intranasal midazolam (Versed) Others Intranasal (IN) Naloxone Background Absorption of IN naloxone almost as fast as IV in both animal and human models Hussain et al, Int J Pharm, 1984 Loimer et al, Int J Addict, 1994 Loimer et al, J Psychiatr Res, 1992 “Atomization” of medications show much better absorption via the IN route Thorsson et al, Br J Clin Pharmacol, 1999 The Denver Experience… Denver Health Paramedic System Administering 600-800 doses of naloxone (Narcan®) a year intravenously to patients Sheathed needles were not used properly No change in incidence of bloodborne exposures “Intranasal Administration of Naloxone by Paramedics” Prospective clinical trial Preliminary study February, 2001 Barton et al, Prehosp Emer Care, 2002 Final study completed August, 2001 Presented at: The First International Congress of Emergency Medicine, Stresa, Italy, September 2001 ACEP Scientific Assembly Research Forum, Chicago, IL, October 2001 Prehospital IN Naloxone Study Purpose To test the efficacy of IN Naloxone in the prehospital setting: Number of IN responders? Time to patient response? How many required repeat doses? Determine what percentage of IV placements could potentially be avoided in the field Prehospital IN Naloxone Methods Clinical indicators for naloxone: “Found down” (FD) “Suspected overdose” (OD) “Altered mental status” (AMS) Patients given 2mg IN naloxone at first contact 1 mg via Mucosal Atomizer Device (MAD) into each nostril (2mg total of 2mg/2ml solution) Same dose as given IV by protocol IN Naloxone by Paramedics Prehospital IN Naloxone Mucosal Atomizer Device (MAD) Single-use Disposable Fits on standard syringe Manufactured by Wolfe-Tory Medical, Inc., Lake City, UT Devices donated for study Salt Mucosal Atomizer Device (MAD) IN Naloxone by Paramedics Prehospital IN Naloxone Methods (cont.) Standard protocols followed including airway management, establish IV, IV meds (naloxone, D50) if needed Medics could discontinue protocols if patient responded appropriately Times of initial patient contact, IN naloxone, IV placement, IV naloxone, and patient response were recorded to nearest minute Prehospital IN Naloxone Results (cont.). 43/52 (83%) = “IN Naloxone Responders.” Mean time = 3.9 minutes (range 1-11 min.). Median time = 3 min. Mean time from first contact = 9.9 min. Median time from first contact = 8 min. 9/52 (17%) = “IN Non-responders.” 4 patients noted to have “epistaxis,” “trauma,” or “septal abnormality.” Prehospital IN Naloxone Results (cont.) IN Naloxone Responders 12/43 (29%) got no IV in the field 7/43 (16%) required additional dose of IV naloxone “leakage from L nares” “aroused slowly” “recurrent somulence” “2mg IV given due to slow response” “lower IN dose - spilled filling syringe” “pt responded within 90-120 sec, but still had decr LOC Prehospital IN Naloxone Conclusions IN naloxone effective route Inexpensive device 83% response in the field MAD May decrease prehospital blood exposures 29% no IV in the field Higher risk populations in this setting Other Naloxone Studies… IV vs. SQ Naloxone: Wanger et al, Acad Emer Med, 1998. 196 patients in Vancouver, BC. IV naloxone (0.4mg) vs. SQ (0.8mg). Response time = crew arrival to RR > 10. Median response time IV = 9.3 min. Median response time SQ = 9.6 min. Conclusions = No significant difference. Delay in SQ response offset by time for IV insertion. *Median response time IN naloxone = 8.0 min. Prehospital IN Naloxone Take away lessons for nasal naloxone: Dose and volume – higher concentration preferred so use 1mg/ml IV solution. Delivery – immediately on decision to treat inject naloxone into nose with MAD, then begin standard care. Successful awakening eliminates the need for any IV or further ALS care. Awakening is gradual, but adequate respiratory efforts occur as fast or faster than IV naloxone due to no delays with IV start. Not 100% effective so failures with IN naloxone need to be followed with IV naloxone. Prehospital IN Midazolam Why intranasal midazolam for seizures in the EMS setting? No needles, no need for an IV in a seizing patient. Rapid delivery – No delays in IV attempts. Socially acceptable: No need for rectal drug administration. IN Midazolam Supporting data: Nasal midazolam has been extensively studied for over a decade with hundreds of studies published regarding its effectiveness for sedating children. Very effective for treating acute seizures and status epilepsy. IN Midazolam Sedation Henry et al, Pediatr Dent, 1998 IV vs. IN drop vs. IN atomized midazolam in dogs Conclusions: Both nasal routes produced higher CSF concentrations; atomized higher than IN drops Malinovsky et al, Anaesthesia, 1995 Oral vs. IN vs. rectal midazolam in kids Conclusions: Sedation occurred sooner with IN meds (7.7min vs. 12.5min rectal) IN Midazolam Seizures. Lahat et al, BMJ, 2000. Prospective study: IN midazolam versus IV diazepam for prolonged seizures (>10 minutes) in children. Similar efficacy in stopping seizures (app. 90%). Time to seizure cessation: IV Valium: 8.0 minutes. IN Versed: 6.1 minutes. IN Midazolam Lahat et al, BMJ, 2000 (cont): Conclusions: IV diazepam and IN midazolam have similar efficacy at controlling prolonged seizures in children. IN midazolam controls seizures more rapidly because there is no delay in establishing an IV. IN Midazolam Sheepers et al, Seizure, 2000. IN midazolam for treatment of severe epilepsy in adults. Results: IN midazolam effective in 94% of seizures. Conclusion: IN midazolam an effective method for controlling seizures and is a “more acceptable and dignified route” than rectal diazepam. IN Midazolam Take away lessons for nasal midazolam: Dose and volume: Higher concentration required use 5mg/ml IV solution. Dosing calculations are difficult: Use a predefined age or weight based table to determine dose. Deliver immediately on decision to treat: Atomize into nose with MAD, then begin standard care. Efficacy: No quite 100% effective so failures with nasal may need follow-up with IV therapy. Other IN Medications ALS Drugs Bleske et al, Am J Emerg Med, 1996 IN epinephrine and phentolamine in dogs during CPR Conclusions: There is a dose response effect with IN epi; IN epi reaches systemic circulation and increases coronary perfusion pressures Jelstad et al, Tidsskr Nor Laegeforen, 1995 Case report: Systemic anticholinergic toxicity after IN intake of atropine Other IN Medications ALS Drugs (cont.) Scavone et al, Br J Clin Pharmacol, 1989 Bioavailability of IN lidocaine gel preparation vs. IV lidocaine in humans Conclusions: IN absorption was less than 50% Pontiroli et al, Diabetes Care, 1989 IN vs. IM glucagon in health adults and IDDM Conclusions: IN glucagon was similar to IM in raising glu levels; IN was quicker than oral glu Other IN Medications ALS Drugs (cont.) Dich-Nielsen et al, Acta Anaesth Scand, 1986 IN NTG on cardiac response to laryngoscopy Conclusions: IN NTG attenuates pressor response to laryngoscopy; more effective than IV lidocaine Hallett et al, Anaesthesia, 2000 Patient-administered IN diamorphine after surgery Conclusions: IN diamorphine is effective and well-tolerated without significant side-effects Conclusions Multiple drugs can be given IN Rapid Immediate access Can be given to almost anyone Exception = Nasal mucosal abnormalities. “Atomization” is the best method Cheap, easy to use device Disposable/single use (MAD) Appropriate drug concentrations Conclusions IN is a true “needleless” system! Reduce Level III bloodborne exposures HIV Hepatitis B, C Decrease IV placements in the field Practice! MAD function: Everyone should familiarize themselves with the MAD before using it in the field. Dosing: IN naloxone: 2 mg (2 ml) – 1 ml up each nostril. IN midazolam: Use highly concentrated formulation (5 mg/ml) Use an age/weight based dosing guide for IN midazolam. Using the MAD 1st: Draw up practice solutions: Saline or water. Be aware of volume and what dose that would equal. 2nd: Expel air from syringe. 3rd: Attach the MAD device via luer lock. 4th: Briskly compress the syringe plunger. Brisk brief compression results in controlled atomization. Gently pushing the plunger will not result in atomization. With practice you can give an exact volume down to 1/10 of a milliliter.