1616 Fort Myer Drive, 12th Floor Arlington, Virginia 22209
advertisement

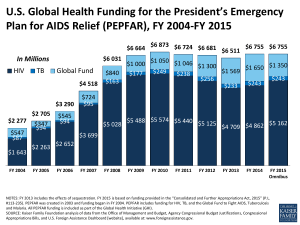
1616 Fort Myer Drive, 12th Floor Arlington, Virginia 22209-3100 USA www.scms.pfscm.org Telephone: +1.571.227.8600 Fax: +1.571.227.8601 SCMS Supply Lines April 2013 Content: Feature Article Trends News Dose The Coordinated Procurement Planning (CPP) Initiative: Going further by working together For quite a while now, almost all news from the global AIDS response has been positive: Praise for PEPFAR from the Institute of Medicine. A UNAIDS report showing great progress in most countries. Large numbers of men circumcised to reduce risk of transmission. An infant effectively cured. Optimism abounds. The international community agrees that credit for this success can be shared among all those working to bring an effective end to the epidemic. Donors to AIDS program have played an obvious role in rapidly scaling up access to prevention, care and treatment programs. Since 2006, key donor groups have been working together through the Coordinated Procurement Planning (CPP) program to prevent gaps, or overlaps in funding, that on one hand could leave programs without necessary funds, and on the other hand could result in unnecessary waste of resources. This global partnership of multilateral institutions, international donors and implementing partners is dedicated to ensuring the uninterrupted flow of ARVs, laboratory commodities, and other essential medicines for HIV/AIDS patients. Together, CPP members join forces to tackle systematic challenges through quarterly meetings and a platform to communicate crucial supply information. How is the CPP structured? General direction of the CPP is guided by the Steering Committee, which plays the key role of defining and monitoring CPP’s strategic goals. The work of CPP is managed through a Technical Working Group with SCMS acting as the Technical Secretariat SCMS Project Team Booz Allen Hamilton | Crown Agents USA, Inc. | i+solutions | JSI Research & Training Institute, Inc. | Management Sciences for Health | The Manoff Group MAP International | North-West University | Northrop Grumman | RTT | UPS Supply Chain Solutions | Voxiva | 3i Infotech Pinpointing risk and preventing crisis “Through the coordinated and concerted effort among CPP Initiative partners, sustainable solutions and emergency supplies provided to countries resulted in better quality of HIV treatment and care through uninterrupted supply of ARVs, diagnostics and other life-saving HIV-related commodities.” – Vincent Habiyambere, Medical Officer, The World Health Organization (WHO) Over the last seven years, CPP members have shared information and actively collaborated to improve procurement coordination and supply planning. This alliance works to identify potential risks to the HIV/AIDS supply chain and works with donors to find solutions to address these risks. The biggest supply chain risk to patients is, of course, stockouts. CPP members agree that a key component to identifying stockout risk is understanding donor funding flows. CPP works to map ARV funding needs against funding availability to help avert this cause of stockouts. In essence, these maps show the money available to the country concerned for ARVs, compared to the distribution and product costs, and illustrate the gap between the two — which must be addressed to prevent treatment interruption. To more clearly assess supply chain issues, CPP developed a Country At Risk Schedule: a stoplight risk analysis and early warning system that tracks country supply and helps prevent concerns becoming crises. Through up-to-date information sharing, the analysis ensures voices of all participating organizations and countries are heard. In March 2013 CPP launched an online information sharing platform available to the CPP members. Snapshot of Quarterly Analysis: This section of the Procurement Information Exchange (PIE) presents country situations as reported to the most recent Steering Committee meetings and a graphic of the average risk levels by country. Clicking on a specific bar in the graphic provides greater detail for the country. The degree of risk shown reflects the reports made to the CPP. Members may export and print customized graphs. PIE — The Procurement Information Exchange The CPP platform — The Procurement Information Exchange (PIE) — serves members to identify countries potentially at-risk from the reports seen by CPP members, and the types of advocacy needed to act on risks. The site hosts an ARV Supply Risk Assessment and other crucial reports. Design and development of this web-based tool was funded by UNITAID and goes beyond data collection and funding stream issues to help members share news about ARV stockout risks. 2 CPP members can access the stoplight risk analysis on the website through the Countries At Risk Schedule’s illustrated graph of countries threatened by treatment interruption. As Technical Secretariat, SCMS updates the site with pertinent content (provided by members), including stockout information and funding needs, gaps and availability, by country. Data is accompanied by quantitative assessments of funding flows relative to needs and a brief qualitative assessment of potential risks. Easy access to this critical information will help donors and partners determine long- and short-term procurement decisions. PIE also displays the ARV Supply Risk Assessment data gathering tool, designed by CPP members to help countries (particularly those at risk of treatment interruption) collect and analyze data on funding flows and ARV stock availability. This tool also offers country focal points another venue to communicate to donor agencies. By connecting all partners involved in procurement, the ARV Supply Risk Assessment aims to help address issues at policy level. SCMS manages the assessment by reporting findings to CPP members and leading mitigation discussions. ART Funding Status for 2013 Snapshot of Funding Status: This tool within PIE generates a customized bar chart showing ARV funding levels by source, country and year. PIE aligns with CPP's mission to provide members an increasingly comprehensive, up-to-date database for ARV procurement coordination. Following the website’s launch, members hope to expand the scope of the platform from ARVs, to include rapid test kits (RTK) and other commodities. Due to the confidential nature of detailed information in PIE the site is currently limited to CPP member organizations and country team members. Through consolidation, cooperation and coordination, CPP has streamlined efforts of multiple partners and offers valuable lessons in collaboration. Members can now research years of data indicators at the click of a mouse. CPP’s role is perhaps more essential now than when it was first created. Seven years ago, funding for AIDS was rapidly increasing from multiple funding sources. In today’s environment of tight budgets, the CPP is helping ensuring that every dollar counts and that the dollars are in the right place when needed. 3 Trends Each month, Supply Lines reports on global trends in HIV/AIDS supply chains. For more information, e-mail SCMSInfo@pfscm.org. Here are some current highlights: Delays in vendor on-time-delivery Significant increases in global demand have resulted in delays in delivery of ARVs. Due to a change in treatment protocols to Tenofovir (TDF)-based treatments manufactured in triple combinations, a number of countries are attempting to implement rapid regimen changes and requesting large orders. Also, high demand from the Government of South Africa as their program grows continues to impact the supply of active pharmaceutical ingredients (API). SCMS anticipated these demand increases and kept clients and vendors informed of potential availability issues. As a result, vendors are now increasing their capacity to produce APIs and finished dosage formulations. Results of increased production should take effect by the second half of 2013. We also understand that at least one additional vendor has applied for (tentative) FDA approval for a triple combination TDF based ARV under assessment; if granted this will enlarging the pool of eligible suppliers. In the meantime, clients should plan for lead times of six months to allow for cost savings from the use of ocean freight rather than costly shipment by air. The WHO has removed notice of concern for SD Bioline In March, the WHO put SD Bioline back on its list of approved HIV RTK suppliers, after removing the kits in November 2011 due to concerns about quality. At this point, USAID approval is pending results of additional testing and final decision by the US Government. Having another approved HIV test kit would benefit the market by increasing diversity. Currently, the global market for HIV RTKs relies on a limited number of suppliers, which presents potential risks should one of these vendors face disruptions to service delivery. SCMS will keep you updated on further developments. News Dose “Visconti’s coup” The Economist, March 14, 2013 March 14 saw the publication of results from the Visconti trial (the name is a contraction of “Virological and immunological studies in controllers after treatment interruption”) and the possibility of using antiretroviral drugs to produce something akin to a cure. http://www.economist.com/blogs/babbage/2013/03/aids-treatment More from Reuters: http://www.reuters.com/article/2013/03/15/us-hiv-functional-cure-idUSBRE92E0C520130315 “PEPFAR’s glowing report card, 10 years later” The Washington Post, February 25, 2013 Hard work is still ahead, and there may be fewer glowing report cards like this one. But Congress and the administration should take note: PEPFAR has changed millions of lives for the better and can do so in the next decade as well. www.washingtonpost.com/opinions/pepfars-glowing-report-card-10-years-later/2013/02/25/1a1c67e4-7ede-11e2b99e-6baf4ebe42df_story.html Read more: http://www.nlm.nih.gov/medlineplus/news/fullstory_134235.html http://www.aidspan.org/gfo_article/pepfar-evaluation-identifies-synergies-and-challenges-working-global-fund 4 “PEPFAR: Addressing Gender and HIV/AIDS” PEPFAR, March 7, 2013 “HIV/AIDS is not just a health issue. It’s a social issue that impacts men and women differently, and it’s an issue linked with and affected by gender inequality. Our success in fighting this epidemic is tied to our ability to recognize and respond to this reality.”-Ambassador Eric Goosby. http://www.pepfar.gov/press/2013/205796.htm “Medicines Patent Pool gets help on HIV/AIDS drug from Glaxo-Pfizer-Shionogi joint venture” Philly Pharma, February 28, 2013 The Medicines Patent Pool announced a new deal with ViiV Healthcare to facilitate greater availability of a key pediatric HIV/AIDS medicine. http://www.philly.com/philly/blogs/phillypharma/Medicines-Patent-Pool-gets-help-on-HIVAIDS-drug-fromGlaxo-Pfizer-Shionogi-joint-venture.html “CROI 2013: 20th Conference on Retroviruses and Opportunistic Infections” AIDSMAP, March 2013 Important news from CROI 2013 includes updates on Cotrimoxazole Profylaxis in children, dual action against HIV and Hep B, a new pro-drug of Tenofovir, and more. http://www.aidsmap.com/croi2013 “PEPFAR report recommends countries control own HIV/AIDS programs” Global Post, March 1, 2013 An independent evaluation of PEPFAR praised the program’s achievements but said its work is unfinished. Among its recommendations is a shift to an advisory role. http://www.globalpost.com/dispatches/globalpost-blogs/global-pulse/pepfar-report-iom-hiv-aids “US gives Burundi US$ 15.7 million to fight HIV/AIDS” Actualité Economie Finances Sports, March 2, 2013 The US will give Burundi an additional $ 3.5 million this year to enable the country to continue its fight against HIV/AIDS and mother-to-child transmission of the disease. http://www.afriquejet.com/201303022950/US-gives-Burundi-US$-15.7-million-to-fight-HIV/AIDS.html. “Routine viral load monitoring almost halves risk of virologic failure in 18-month Kenyan study” AIDSMAP, March 12, 2013 Six-monthly viral load testing of patients taking antiretroviral therapy (ART) at primary health clinics in rural Kenya reduced the risk of virologic failure at 18 months of follow-up by 46 percent, according to the results of the Clinic-based ART Diagnostic Evaluation. http://www.aidsmap.com/Routine-viral-load-monitoring-almost-halves-risk-of-virologic-failure-in-18-monthKenyan-study/page/2596417/?utm_source=NAM-Email-Promotion&utm_medium=aidsmapnews&utm_campaign=aidsmap-news 5