File - Michels Academy
advertisement

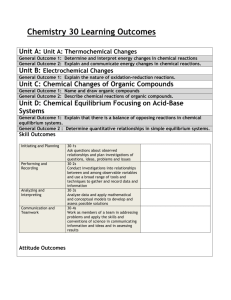
SCH4U Course Outline Course Description This course enables students to deepen their understanding of chemistry through the study of organic chemistry, energy changes and rates of reaction, chemical systems and equilibrium, electrochemistry, and atomic and molecular structure. Students will further develop problemsolving and laboratory skills as they investigate chemical processes, at the same time refining their ability to communicate scientific information. Emphasis will be placed on the importance of chemistry in daily life, and on evaluating the impact of chemical technology on the environment. Units of Study Scientific Investigation Skills and Career Exploration A1. demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and planning, performing and recording, analysing and interpreting, and communicating); A2. identify and describe careers related to the fields of science under study, and describe the contributions of scientists, including Canadians, to those fields. Organic Chemistry B1. assess the social and environmental impact of organic compounds used in everyday life, and propose a course of action to reduce the use of compounds that are harmful to human health and the environment; B2. investigate organic compounds and organic chemical reactions, and use various methods to represent the compounds; B3. demonstrate an understanding of the structure, properties, and chemical behaviour of compounds within each class of organic compounds. Structure and Properties of Matter C1. assess the benefits to society and evaluate the environmental impact of products and technologies that apply principles related to the structure and properties of matter; C2. investigate the molecular shapes and physical properties of various types of matter; C3. demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. Energy Changes and Rates of Reaction D1. analyse technologies and chemical processes that are based on energy changes, and evaluate them in terms of their efficiency and their effects on the environment; D2. investigate and analyse energy changes and rates of reaction in physical and chemical processes, and solve related problems; D3. demonstrate an understanding of energy changes and rates of reaction. Chemical Systems and Equilibrium E1. analyse chemical equilibrium processes, and assess their impact on biological, biochemical, and technological systems; E2. investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems; E3. demonstrate an understanding of the concept of dynamic equilibrium and the variables that cause shifts in the equilibrium of chemical systems. Electrochemistry F1. analyse technologies and processes relating to electrochemistry, and their implications for society, health and safety, and the environment; F2. investigate oxidation-reduction reactions using a galvanic cell, and analyse electrochemical reactions in qualitative and quantitative terms; F3. demonstrate an understanding of the principles of oxidation-reduction reactions and the many practical applications of electrochemistry. Units of Study Unit Subject No. of hours Evaluation 1 Structure and Properties of Matter 16 Unit Test 2 Energy Changes and Rates of Reaction 18 Unit Test 3 Chemical Systems and Equilibrium 30 Unit Test 4 Electrochemistry 18 Unit Test 5 Organic Chemistry 16 Unit Test 1. Organic Compounds ................................................................................... 2. Atoms, Electrons and Periodic Trends...................................................... 3. Structures and Properties of Substances........……............................… 4. Energy and Change and Rates of Chemical Reactions .................…….. 5. Reversible Reactions and Chemical Equilibrium............................……… 6. Acids, Bases and pH...................................................................................... 7. Aqueous Solutions and Solubility Equilibria..............................…………….... 8. Electrochemistry.......................................................................................... 9. Culminating: Course Challenge..................................................................... 10. Inquiry: Best 4 out of 8............................................................................... Summative Evaluations 11. Midterm Evaluation/ Prep................... 5 hours 12. Final Evaluation/ Prep......................... 5 hours Achievement Categories/Strands Knowledge / Understanding 35% Thinking 15 % Application 35% Communication 15% 11 hours 11 hours 11 hours 11 hours 11 hours 11 hours 11 hours 11 hours 8 hours 4 hours Assessment and Evaluation Strategies In order to ensure that assessment and evaluation are valid and reliable, and lead to improvement of student learning, teachers of this course use a variety of the following strategies to assess student learning and to provide them with feedback: teacher observation oral presentations, interviews essays, reports, reviews, critiques, letters, journals, creative writing, computer lab work media works quizzes, tests, examinations performance tasks, dramatic presentations portfolios, design projects, lab work self-assessment, peer assessment check lists, rubrics questions and answers Some of these strategies are also used for evaluation. However, evaluation is the responsibility of the teacher and is based on individual student demonstration of course expectations. Evaluated group tasks likewise must reflect individual accountability for learning and demonstration of course expectations through work submitted. SCH4U Course Mark Breakdown 8 Tests worth 52% in total Course Challenge worth 14% Inquiries worth 4% 1 Mid-Term Evaluation worth 10% 1 Final Evaluation worth 20% Evaluation Mark Workbook Content Sem 1 Sem 2 Tentative Dates TESTS 1 & 2 14% Chapters 1 & 2 TESTS 3 & 4 14% Chapter 3 & 4 TEST 5 & 6 14% Chapter 5 & 6 TEST 7 & 8 14% Chapter 7 & 8 Inquiries 4% Culminating Activity 14% Teacher Assigned Online Quizzes, Dropboxes, Textbook Assignment Questions diagnostic TBA Mid-Term Exam 30% Final Exam Chapter 1 to 8 TBA The Report Card: The report card will focus on two distinct but related aspects of student achievement; the achievement of curriculum expectations and the development of learning skills. The report card will contain separate sections for the reporting of these two aspects. A Summary Description of Achievement in Each Percentage Grade Range and Corresponding Level of Achievement Percentage Achievement Grade Level Range Summary Description 80-100% Level 4 A very high to outstanding level of achievement. Achievement is above the provincial standard. 70-79% Level 3 A high level of achievement. Achievement is at the provincial standard. 60-69% Level 2 A moderate level of achievement. Achievement is below, but approaching, the provincial standard. 50-59% Level 1 A passable level of achievement. Achievement is below the provincial standard. below 50% Level R Insufficient achievement of curriculum expectations. A credit will not be granted. Overall Expectations: SCH4U Scientific Investigation Skills and Career Exploration Overall Expectations demonstrate scientific investigation skills (related to both inquiry and research) in the four areas of skills (initiating and 150.040.01.01 planning, performing and recording, analysing and interpreting, and communicating); identify and describe a variety of careers related to the fields 150.040.01.02 of science under study, and identify scientists, including Canadians, who have made contributions to those fields. Organic Chemistry Overall Expectations assess the social and environmental impact of organic compounds used in everyday life, and propose a course of 150.040.02.01 action to reduce the use of compounds that are harmful to human health and the environment; investigate organic compounds and organic chemical 150.040.02.02 reactions, and use various methods to represent the compounds; demonstrate an understanding of the structure, properties, 150.040.02.03 and chemical behaviour of compounds within each class of organic compounds. Structure and Properties of Matter Overall Expectations assess the benefits to society and evaluate the environmental 150.040.03.01 impact of products and technologies that apply principles related to the structure and properties of matter; 150.040.03.02 investigate the molecular shapes and physical properties of various types of matter; demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical 150.040.03.03 properties of ionic, molecular, covalent network, and metallic substances. Energy Changes and Rates of Reaction Overall Expectations analyse technologies and chemical processes that are based 150.040.04.01 on energy changes, and evaluate them in terms of their efficiency and their effects on the environment; investigate and analyse energy changes and rates of reaction 150.040.04.02 in physical and chemical processes, and solve related problems; 150.040.04.03 demonstrate an understanding of energy changes and rates of reaction. Chemical Systems and Equilibrium Overall Expectations 150.040.05.01 analyse chemical equilibrium processes, and assess their impact on biological, biochemical, and technological systems; 150.040.05.02 investigate the qualitative and quantitative nature of chemical systems at equilibrium, and solve related problems; demonstrate an understanding of the concept of dynamic 150.040.05.03 equilibrium and the variables that cause shifts in the equilibrium of chemical systems. Electrochemistry Overall Expectations analyse technologies and processes relating to 150.040.05.01 electrochemistry, and their implications for society, health and safety, and the environment; investigate oxidation-reduction reactions using a galvanic 150.040.05.02 cell, and analyse electrochemical reactions in qualitative and quantitative terms; demonstrate an understanding of the principles of oxidation150.040.05.03 reduction reactions and the many practical applications of electrochemistry. Classtime Expectations The goal of this course is to prepare you for university level studies, both academically and emotionally. Success at university requires a high degree of maturity, responsibility, and independence, all skills that must be developed prior to university admission. To this end, students in this class must demonstrate the ability to contribute productively to the classroom environment. All members of the class must meet the following expectations. 1. Electronic Devices Required The Michels Academy is designed to be a technology school. Laptops are expected to be part of your course materials. You will be working, more times than less, in the online learning environment (LE) being that all of the course material is provided for you . For safety, it is recommended that these devices not be left unattended. The Oshawa classroom and most libraries are wireless environments. Cell phones provide easy access to the Internet and text messaging between students. As such, they are prohibited during tests and exams to prevent cheating. The privacy, dignity and safety of others must be maintained through the appropriate use of cell phones and electronic devices. 2. Attendance All students are expected to attend class on a daily basis. Any absences must be justified with a note from a parent or doctor. If a student misses a class, it is his or her responsibility to make up any missed work. This should be done through discussion with classmates and consultation of the Michels Academy. Use the TOLL FREE phone number 856-677-3669 to leave a voice message or send an email to michelsacademy@hotmail.com 3. Punctuality All students are expected to arrive to class on time in accordance with the school schedule. Classes begin at the top of the hour. Classtime is typically 2 hours with the teacher and 1 to 1.5 hours in the before or after classtime working within the LE. Lateness disrupts the classroom environment and is disrespectful to all members of the class. 4. Assignments & Deadlines It is crucial that students learn to complete assignments by a deadline if they wish to succeed in university. Students will be given ample time to complete all required assignments. Due dates for assignments will be set in advance, and will also be available on the course website. Assignments must be handed in at the beginning of the class on the day on which they are due. Students who fear that they will be unable to meet these deadlines must conference with the teacher in advance of the due date.