Standard Enthalpy of Formation and Reaction
advertisement
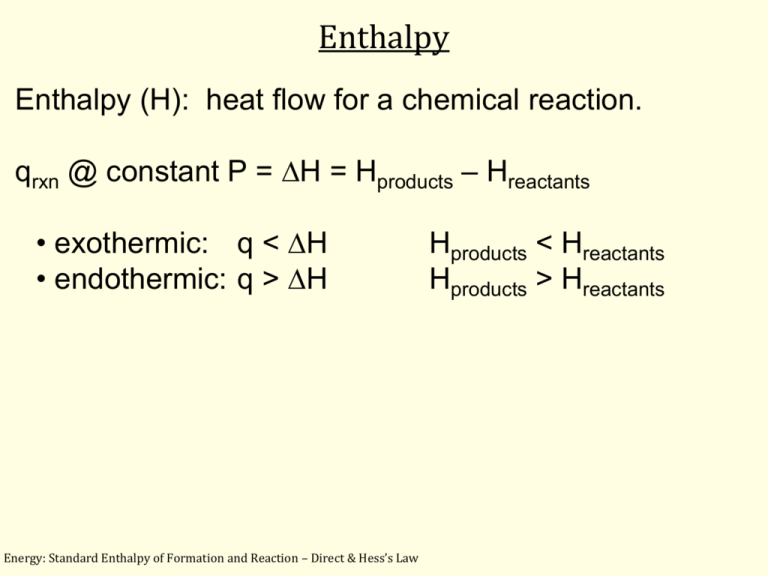
Enthalpy Enthalpy (H): heat flow for a chemical reaction. qrxn @ constant P = DH = Hproducts – Hreactants • exothermic: q < DH • endothermic: q > DH Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Hproducts < Hreactants Hproducts > Hreactants Standard Enthalpy of Formation and Reaction Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Standard Enthalpy of Formation and Reaction aA + bB → cC + dD standard-state conditions = 25oC & 1 atm Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.10 Standard Enthalpy of Reactions: Direct Method 2Al(s) + 1Fe2O3(s) → 1Al2O3(g) + 2Fe(l) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.10 (con’t) 2Al(s) + 1Fe2O3(s) → 1Al2O3(g) + 2Fe(l) Al(s) Fe(l) 0 kJ/mol 12.40 kJ/mol Al2O3(s) –1669.8 kJ/mol Fe2O3(s) H = 0 kJ/mol for elements at 25oC (e.g., Fe(s) = 0 kJ/mol; Fe(l) 0) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law –822.2 kJ/mol Example 6.10 (con’t) 2Al(s) + 1Fe2O3(s) → 1Al2O3(g) + 2Fe(l) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law INDIRECT METHOD (Hess’s Law) Hess’s Law is used is convenient for obtaining values of DH for reactions that are difficult to carry out in a calorimeter. E.g., C(s) + ½O2(g) CO(g) Nearly impossible to carry out in a calorimeter because when C is burned, CO2 is almost always produced. For H2 + Cl2 2HCl DH = –185 kJ • –185 kJ..............sign = exothermic • coefficients represent moles (the -185 kJ refers to ONE MOLE of reaction) • states of matter (phases) MUST be written • standard temp = 25oC (not STP: 0oC) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Rules: (For H2 + Cl2 2HCl DH = –185 kJ 1. Magnitude of DH is directly proportional to the amount of reactant or product. -185 kJ 1 mol H2 -185 kJ 1 mol Cl2 -185 kJ 2 mol HCl 2. Reverse the sign for DH for the reverse reaction. 3. DH for a reaction is the same, whether carried out in one step or several (State Function) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) ΔH kJ/mol (a) C(graphite) + O2(g) →CO2(g) –393.5 (b) H2(g) + ½O2(g) → H2O(l) –285.8 (c) 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) –2598.8 The values for H are determined by experiment, so they need to be given to you; either directly or in a table. Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) ΔH kJ/mol (a) C(graphite) + O2(g) → CO2(g) –393.5 (b) H2(g) + ½O2(g) → H2O(l) –285.8 (c) 2C2H2(g) + 5O2(g)→ 4CO2(g) + 2H2O(l) –2598.8 Concentrate on aligning the substances in the step reactions to be on the same sides as the original equation. Notice: There’s 2 moles of C(graphite) in the original equation but only 1 mole in (a).......................... Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) (2a) (b) (c) 2C(graphite) + 2O2(g) → 2CO2(g) H2(g) + ½O2(g) → H2O(l) ΔH kJ/mol –787.0 –285.8 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) –2598.8 Double (a) to match the original equation. And ΔH doubles, too (–393.5 * 2 = –787.0 kJ/mol) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) (2a) (b) (c) 2C(graphite) + 2O2(g) → 2CO2(g) H2(g) + ½O2(g) → H2O(l) ΔH kJ/mol –787.0 –285.8 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) –2598.8 H2 is a reactant in both the original equation and in step (b). And, there’s 1 mole H2 in the original equation and 1 mol H2 in step (b). Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) (2a) (b) (c) 2C(graphite) + 2O2(g) → 2CO2(g) H2(g) + ½O2(g) → H2O(l) ΔH kJ/mol –787.0 –285.8 2C2H2(g) + 5O2(g) → 4CO2(g) + 2H2O(l) –2598.8 Align (c) C2H2 with original equation as a product by reversing step (c) .......... Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) (2a) (b) (–c) 2C(graphite) + 2O2(g) → 2CO2(g) H2(g) + ½O2(g)→ H2O(l) ΔH kJ/mol –787.0 –285.8 4CO2(g) + 2H2O (l)→ 2C2H2(g) + 5O2(l) +2598.8 Reverse (c) so it on the same side as the original equation. Include reversing the sign of ΔH, too. Shown by (–c). However, there 2 moles of C2H2 in step (c) but only 1 mole in the original equation. So, we need to halve step (c).......................................................... Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) (2a) (b) 2C(graphite) + 2O2(g) → 2CO2(g) H2(g) + ½O2(g) → H2O(l) ΔH kJ/mol –787.0 –285.8 Divide step (–c), including ΔH, by 2 to match the original equation’s 1 mole C2H2. (shown by (–c/2) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law (2a) 2C(graphite) + 1H2(g) → 1C2H2(g) ΔH kJ/mol 2C(graphite) + 2O2(g) → 2CO2(g) –787.0 (b) 1H2(g) + ½O2(g) → 1H2O(l) O2: 2+½ → CO2: H2O: –285.8 5/2 2 → 2 1 1 H = 226.6 kJ/mol Determine the net equation for both substances & ΔH by adding them together. Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) (2a) (b) (-c/2) 2C(graphite) ΔH kJ/mol –787.0 + 1H2(g) –285.8 → 1C2H2(g) 2C(graphite) + 1H2(g) → 1C2H2(g) +1299.4 +226.6 kJ/mol Net equation of steps = original equation H = +226.6 kJ/mol Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law Example 6.09 Standard Enthalpy of Reactions: Hess’s Law 2C(graphite) + 1H2(g) → 1C2H2(g) Energy: Standard Enthalpy of Formation and Reaction – Direct & Hess’s Law ΔH = +226.6 kJ/mol Fin 330_06_05 Energy: Calorimetry




