Experiment 1: Calibration of and Choosing Glassware
advertisement
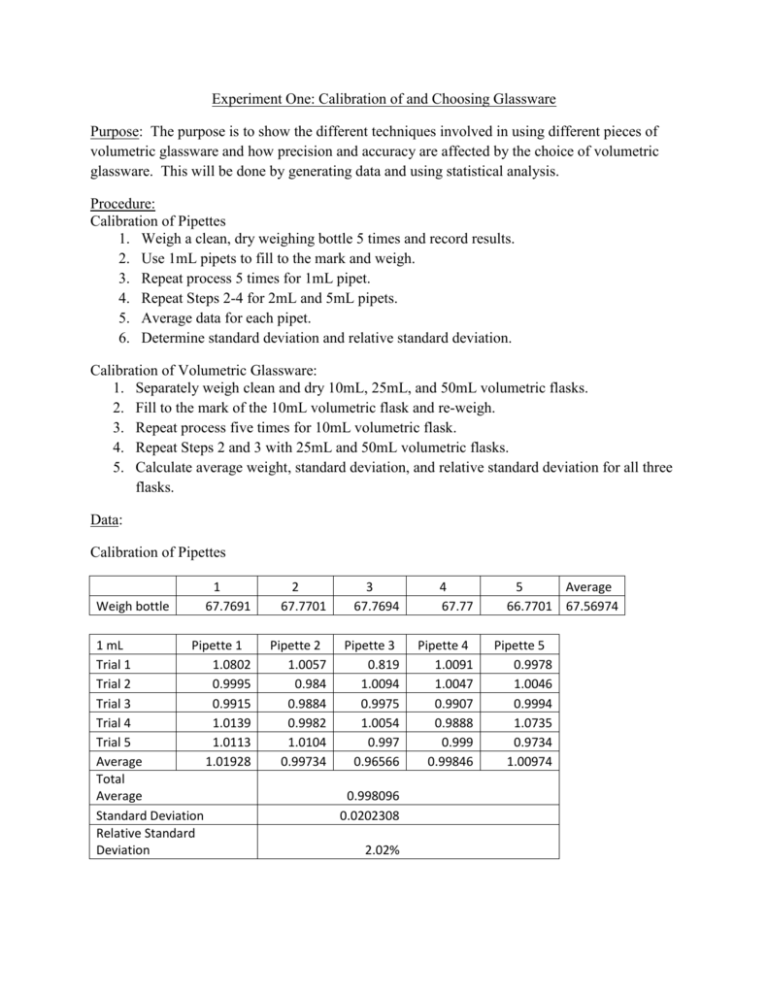
Experiment One: Calibration of and Choosing Glassware Purpose: The purpose is to show the different techniques involved in using different pieces of volumetric glassware and how precision and accuracy are affected by the choice of volumetric glassware. This will be done by generating data and using statistical analysis. Procedure: Calibration of Pipettes 1. Weigh a clean, dry weighing bottle 5 times and record results. 2. Use 1mL pipets to fill to the mark and weigh. 3. Repeat process 5 times for 1mL pipet. 4. Repeat Steps 2-4 for 2mL and 5mL pipets. 5. Average data for each pipet. 6. Determine standard deviation and relative standard deviation. Calibration of Volumetric Glassware: 1. Separately weigh clean and dry 10mL, 25mL, and 50mL volumetric flasks. 2. Fill to the mark of the 10mL volumetric flask and re-weigh. 3. Repeat process five times for 10mL volumetric flask. 4. Repeat Steps 2 and 3 with 25mL and 50mL volumetric flasks. 5. Calculate average weight, standard deviation, and relative standard deviation for all three flasks. Data: Calibration of Pipettes 1 67.7691 2 67.7701 3 67.7694 4 67.77 1 mL Pipette 1 Trial 1 1.0802 Trial 2 0.9995 Trial 3 0.9915 Trial 4 1.0139 Trial 5 1.0113 Average 1.01928 Total Average Standard Deviation Relative Standard Deviation Pipette 2 1.0057 0.984 0.9884 0.9982 1.0104 0.99734 Pipette 3 0.819 1.0094 0.9975 1.0054 0.997 0.96566 Pipette 4 1.0091 1.0047 0.9907 0.9888 0.999 0.99846 Weigh bottle 0.998096 0.0202308 2.02% 5 Average 66.7701 67.56974 Pipette 5 0.9978 1.0046 0.9994 1.0735 0.9734 1.00974 2 mL Pipette 1 Pipette 2 Pipette 3 Pipette 4 Pipette 5 Trial 1 1.9816 1.9703 1.9859 1.9987 1.9901 Trial 2 1.9978 1.9341 1.9665 1.9933 1.9918 Trial 3 1.9820 1.9934 1.9693 1.9957 1.9934 Trial 4 1.9989 1.9893 1.9458 1.8999 1.9889 Trial 5 1.9900 1.976 1.8722 1.9959 1.9893 Average 1.99006 1.97262 1.94794 1.9767 1.9907 Total Average 1.975604 Standard Deviation 0.0174088 Relative Standard Deviation 0.88% 5 mL Pipette 1 Pipette 2 Pipette 3 Pipette 4 Pipette 5 Trial 1 4.9416 4.9394 4.9395 4.9528 4.9725 Trial 2 4.9348 4.9393 4.9333 4.9601 4.9701 Trial 3 4.9689 4.9320 4.9471 4.9457 4.9598 Trial 4 4.9367 4.9251 4.9458 4.9492 4.9628 Trial 5 4.9302 4.9385 4.9298 4.9511 4.9587 Average 4.94244 4.93486 4.9391 4.95178 4.96478 Total Average 4.946592 Standard Deviation 0.011924 Relative Standard Deviation 0.24% Calibration of Volumetric Glassware Empty Volumetric Glass 10 ml 25 mL 50 mL Trial 1 Trial 2 Trial 3 Trial 4 Trial 5 Average 13.2727 13.2725 13.273 13.2728 13.2724 13.27268 18.3467 18.3476 18.3476 18.3471 18.3475 18.3473 35.639 35.6392 35.6384 35.6383 35.6382 35.63862 10 mL Pipette 1 Pipette 2 Pipette 3 Pipette 4 Pipette 5 Trial 1 9.9993 9.9979 9.9993 9.9989 9.9813 Trial 2 10.001 9.9885 10.0035 9.9992 9.9927 Trial 3 9.9895 9.9691 9.9788 9.9833 9.9948 Trial 4 9.9788 9.9413 9.9499 10.0002 9.9981 Trial 5 9.9900 9.9831 9.9639 9.9993 9.9972 Average 9.99172 9.97598 9.97908 9.99618 9.99282 Total Average 9.987156 Standard Deviation 0.0090065 Relative Standard Deviation 0.09% 25 mL Pipette 1 Pipette 2 Pipette 3 Pipette 4 Pipette 5 Trial 1 24.88 24.8346 24.8823 24.8812 24.9196 Trial 2 24.9086 24.8823 24.8917 24.8897 24.9075 Trial 3 24.8912 24.9033 24.9039 24.8791 24.8955 Trial 4 24.9034 24.8911 24.8859 24.8901 24.8897 Trial 5 24.8783 24.8875 24.8958 24.8832 24.8916 Average 24.8923 24.87976 24.89192 24.88466 24.90078 Total Average 24.88988 Standard Deviation 0.0080388 Relative Standard Deviation 0.03% 50 mL Pipette 1 Pipette 2 Pipette 3 Pipette 4 Pipette 5 Trial 1 49.4655 49.8633 49.6750 49.8001 49.5824 Trial 2 49.6820 49.7238 49.6002 49.7984 49.6276 Trial 3 49.6036 49.7495 49.6489 49.7321 49.5785 Trial 4 49.5941 49.8205 49.7068 49.6992 49.7411 Trial 5 49.4992 49.6912 49.7134 49.7612 49.6503 Average 49.56888 49.76966 49.66886 49.7582 49.63598 Total Average 49.68032 Standard Deviation 0.0845032 Relative Standard Deviation 0.17% Calculations: 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝑊𝑒𝑖𝑔ℎ𝑡 = 𝑥1 + 𝑥2 + 𝑥3 + 𝑥4 + 𝑥5 5 Ex. 1mL Pipet#1: 1.0802 + 0.9995 + 0.9915 + 1.0139 + 1.0113 = 1.01928 5 (𝑥1 − 𝑎𝑣𝑔)2 + (𝑥2 − 𝑎𝑣𝑔)2 + (𝑥3 − 𝑎𝑣𝑔)2 + (𝑥4 − 𝑎𝑣𝑔)2 + (𝑥5 − 𝑎𝑣𝑔)2 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 = √ 𝑛−1 Ex. 1mL Pipet: (1.01928 − 0.998096)2 + (0.99734 − 0.998096)2 + (0.96566 − 0.998096)2 + (0.99846 − 0.998096)2 + (1.00974 − 0.998096)2 √ 4 = 0.0202308 𝑅𝑒𝑙𝑎𝑡𝑖𝑣𝑒 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛𝑛 = 𝑆𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝐷𝑒𝑣𝑖𝑎𝑡𝑖𝑜𝑛 × 100% 𝐴𝑣𝑒𝑟𝑎𝑔𝑒 𝑊𝑒𝑖𝑔ℎ𝑡 Ex. 1mL Pipet: 0.0202308 × 100% = 2.02% 1.01928 Conclusion: For this lab, different sized pipettes and volumetric glassware were tested for their accuracy using water. The results of this experiment showed that as the size of glassware or pipette increased, the standard of deviation decreased. This proved that the larger the glassware or pipette, the more accurate it is. For example, the calculated standard deviation of the 5mL pipette was 0.01194, which was smaller than the calculated standard deviation of the 1mL pipette, which was 0.020238. Overall, the volumetric flasks were more accurate than the pipettes. Human error, such as extra water left in the flask or an inaccurate measurement of the pipette, could attest for the abnormal standard deviation calculation for our 50mL volumetric flask. After-Lab Questions: 1. Explain using calculations and words whether it is better to use 20, 49, or 56 mL of solution from a fifty mL buret. It would be better to use the 49 mL of solution from a fifty mL buret. The 20 mL would not be better since, as our calculations showed, you want the buret to be filled as much as possible with as little chance of error. Our calculations showed that the bigger the buret, the smaller the relative deviation and therefore, less error associated with the measurements. It also would not be better to use 56 mL of solution since it would be challenging to have to measure out 50 mL of solution and then add another 6 mL without some form of human error affecting the results. 2. Assuming the volume of base used had a mean value of 43.56mL with a std dev of 0.89mL using five titrations, the molarity of base was 0.1012M std dev 0.0025, and the volume of acid had a mean value of 50mL std dev 0.05mL, what would be the calculated molarity and error associated with this measurement? 𝑉𝑏𝑎𝑠𝑒 × 𝑀𝑏𝑎𝑠𝑒 = 𝑉𝑎𝑐𝑖𝑑 × 𝑀𝑎𝑐𝑖𝑑 (43.56𝑚𝐿) × (0.1012𝑀) = (0.050𝐿 × 1000𝑚𝐿) × 𝑀𝑎𝑐𝑖𝑑 𝑀𝑎𝑐𝑖𝑑 = 0.088𝑀 % 𝑒𝑟𝑟𝑜𝑟 = √[ 0.89 × 100 2 0.0025 × 100 2 0.05 × 100 2 ] +[ ] +[ ] 43.56 0.1012 50 % 𝑒𝑟𝑟𝑜𝑟 = 3.21%