The Nature of Light
advertisement

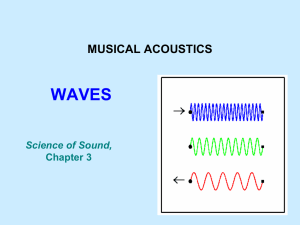
General Astronomy The Nature of Light Our ideas today on the nature of light are inductive. That is, from observations we build a (set of) model(s) which explain the observations in a consistant fashion. We begin by looking at some everyday observations. Observation: Reflection We see reflections everywhere. From the bathroom mirror in the morning, to shiny objects to moonlight on the ocean at night. The Rule: The angles in and out are equal Note that all colors reflect at the same angle. Observation: Refraction Refraction is the bending of light when it passes between two different media. For example, water and air. Light will bend toward the normal when moving from a less to greater 'index of refraction' (usually denser material) Observation: Refraction Different colors refract at different angles Refract light through lots of raindrops and you get a rainbow All colors reflect at the same angle Observation: Color • Most of us see color. Our vision ranges from violet (4000Å) to deep red (7000Å) – One Angstrom (Å) is 1x10-8 cm Corpuscular Model (Particle Model) Reflection Refraction Particle Model Light travels through a vacuum Color Particle Model Reflection Particles "bounce" Angle of incidence = Angle of reflection Refraction Particles slow down in denser material Observation: Scattering • Why is the sky blue? • Why are the clouds white? • Why are sunsets red? Observation: Scattering Blue light is scattered all about the sky so that where ever we look we see the blue color Observation: Scattering Corpuscular Model (Particle Model) Reflection Scattering Light travels through a vacuum Refraction Particle Model Color Observation: Polarization Certain crystals and minerals show curious behavior under some circumstances. It was noticed that looking at light reflected off of water through these crystals brightened and dimmed as the crystal was rotated in front of the eye We are having a problem with our model. How can particles exhibit this kind of behavior? Observation:Diffraction Suppose we shine a light through a narrow opening in a screen, such as sunlight coming through an opening in a shade. We expect to see a bright area pretty much in the same shape as the opening itself. Top View Looking at the screen Diffraction In many cases, it's more useful to show this as a plot of Intensity (brightness) versus position Let's close down the slit … Intensity position Single Slit Diffraction 1 As the slit narrows, instead of the band of light simply getting narrower in proportion, it starts forming bands of light – Diffraction Fringes This is also called a Diffraction Pattern The spacing of the fringes depends on the wavelength and the slit width 0.8 0.6 0.4 0.2 -0.4 -0.2 0.2 0.4 Diffraction This is hard to explain using the particle model of light. If it works for a thin slit, what about a pinhole? Circular Diffraction Passing light through a small hole, produces this kind of pattern. Note that the central fringe is much, much greater than the outer ones. 0.5 0.4 0.3 0.2 0.1 -0.4 -0.2 0.2 0.4 10 8 6 4 2 -0.4 -0.2 0.2 0.4 Straight Edge Diffraction Suppose we block half the light with a straight edge We expect to see a sharp shadow Instead we see a diffraction pattern … 1.2 1 0.8 0.6 0.4 0.2 0.5 1 1.5 2 Observation: Interference What happens if two waves interact with each other? You can get some pretty complicated ripple patterns if you tap two fingers on the water surface. We can do the same with light if we put two slits near each other: Interference Slit separation is 4 times the slit width The Model Diffraction Reflection Scattering Light travels through a vacuum Refraction Particle Model Polarization Color Interference The Wave Model • There is another model that might be used to explain the observations. • What if light were a wave instead of a particle? • First, what do we mean by a wave? • A wave is a disturbance in a medium. – Water waves (ripples displacing the water surface) – Sound waves (rarifications and compressions in air) Wave Properties The amplitude is how "big" the wave gets A wavelength is one repetition of the pattern C = f Speed of Light = (Wavelength)(Frequency) The Wave Model Diffraction Reflection Refraction Scattering Light travels through a vacuum Wave Model Polarization Color Interference Reflection, Refraction & Polarization The Wave Model • Everything we have observed can be explained using waves instead of particles – except one – If a wave is a disturbance in a medium, what is the medium in a vacuum? – It was well known that light could travel through a vacuum, but it is hard to "ripple". – The Aether was invented. • This was an incredibly tenuous medium filling all space • It supported the high speed of light, but did not put an appreciable drag on the planets passing through it. Models • At this point, the wave model can explain most of the observations, it can predict the presence of other new observations. • Light now appears to be "just another" aspect of Electromagnetic waves. Electromagnetic Waves Status • We are now close to the turn of the century (1900 that is). • The wave theory is becoming more entrenched and can explain more and more phenomena. • The particle model is very much in disfavor. • Equipment and measurements are getting more and more accurate. • Maxwell's Laws predict much of electromagnetism and electromagnetic waves --- except for what produces them. Interlude And God said, And there was Light More Observations There were four more observations and experiments which are very important – – – – The Doppler Effect The Photoelectric Effect Blackbody Radiation The Michelson-Morley Experiment The Doppler Effect • Light will shift color depending on the speed of the light source. • Only motion toward or away from you causes this effect; there is no color shift for 'sideways' movement. • Motion toward you shifts the light toward the blue end of the spectrum • Motion away from you shifts the light toward the red end of the spectrum Consider a stationary source sending out light pulses This time the light source moves to the right as it pulses The Doppler Effect Observer sees redder light (the wave crests are farther apart) A B Observer sees bluer light (the wave crests are closer together) Blackbody Radiation Think about heating an old cast-iron frying pan. First, you can feel the heat from the pan Next, you can see a dull red glow Then it's cherry red Then orange, yellow, white Finally it becomes blue-white The color is an indicator of the temperature >30,000 °K 6000 °K 4000 °K 3000 °K Blackbody Radiation Release of an infinite amount of light at short wavelengths was known as the “Ultraviolet Catastrophe” Max Planck postulated in 1900 that light X-rays, and other waves (i.e. energy) can only be emitted or absorbed in discrete amounts which he called quanta (the plural of "quantum", the Latin word for "how much"). The energy quantum is related to the frequency of the wave by a new fundamental constant h The Photoelectric Effect Under certain circumstances, light falling on a metal releases electrons • The energy of the electrons is linearly proportional to the frequency of the light • There will be no electrons if the light is below a certain frequency • The amount of electron flow is proportional to the intensity of the light. The Photoelectric Effect Einstein, using Planck’s idea of a quanta, related the energy of a quanta – or photon – to it’s frequency. The bluer the light, the higher the energy and the more capable of ‘knocking electrons’ out of the metal. The Michelson-Morley Experiment In the late 1890's, an attempt was made to measure the motion of the Earth through the 'luminiferous aether' An interferometer was designed to detect the slightest difference in the distance light travels between two separate paths. As the Earth moves, one expects the path lengths to change depending on if they are going with or across Incoming light the flow of the aether Resultant light There is no measurable change Which Model ? Interference Diffraction Reflection Refraction Photoelectric Color Wave Model Scattering Blackbody Polarization Doppler Particle Model Light travels through a vacuum Which Model ? • Both! • It's called the "Wave-Particle Duality" • It is a model - A view of how it might work. There is no reason why there cannot be several equally valid models. We simply choose the one in which predictions are simplest for a given observation. Wave-Particle Duality Of course, if waves (light) sometimes acts as if it were a particle (called a photon) then do particles (electrons, neutrons, etc.) sometimes act as if they were waves? YES! Electron microscopes, Electron diffraction are used to probe the very small structures of nature. Electrons diffract, interfere and exhibit wave behavior under the right conditions.