Section 16.4-16.8
advertisement
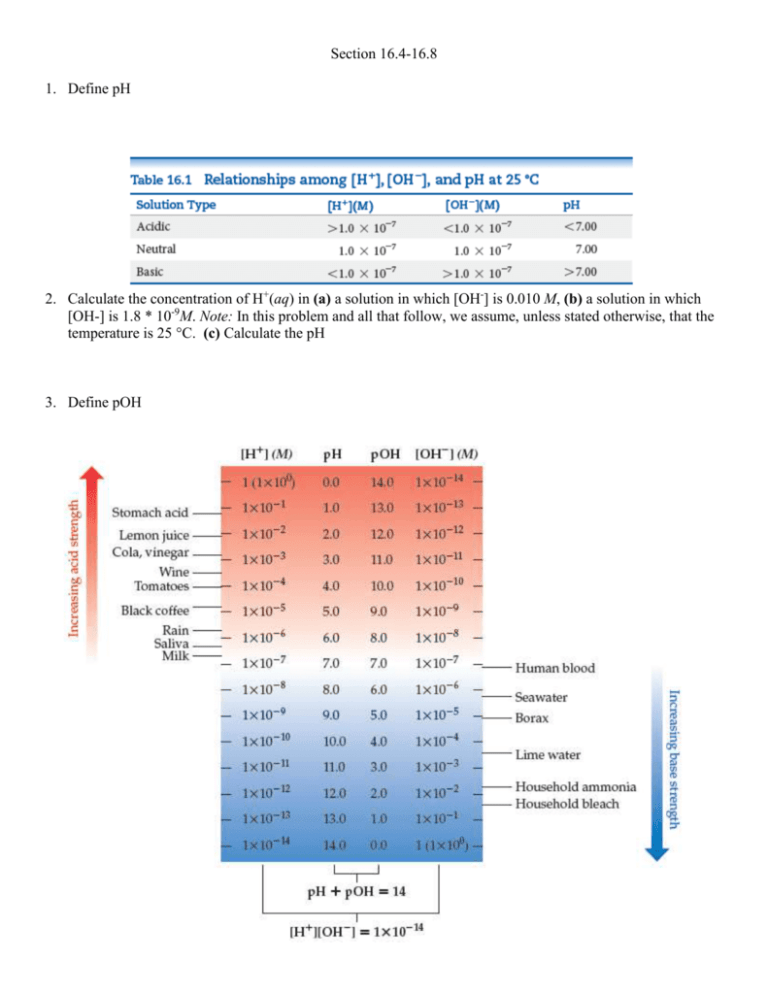
Section 16.4-16.8 1. Define pH 2. Calculate the concentration of H+(aq) in (a) a solution in which [OH-] is 0.010 M, (b) a solution in which [OH-] is 1.8 * 10-9M. Note: In this problem and all that follow, we assume, unless stated otherwise, that the temperature is 25 °C. (c) Calculate the pH 3. Define pOH 4. A sample of freshly pressed apple juice has a pOH of 10.24. Calculate [H+]. 5. How is pH measured? 6. What is a strong acid/base 7. What is the pH of a 0.040 M solution of HClO4? 8. What is the pH of (a) a 0.028 M solution of NaOH, (b) a 0.0011 M solution of Ca(OH)2? 9. Define weak acid/base 10. Define acid-dissociation constant 11. A student prepared a 0.10 M solution of formic acid (HCOOH) and found its pH at 25 °C to be 2.38. Calculate Ka for formic acid at this temperature. 12. Define percent ionization 13. As calculated in Sample Exercise 16.10, a 0.10 M solution of formic acid (HCOOH) contains 4.2 * 10-3 M H+(aq). Calculate the percentage of the acid that is ionized. 14. Calculate the pH of a 0.20 M solution of HCN. (Refer to Table 16.2 or Appendix D for the value of Ka.) 15. Calculate the pH and percentage of HF molecules ionized in a 0.10 M HF solution. 16. Define polyprotic acids 17. The solubility of CO2 in water at 25 °C and 0.1 atm is 0.0037 M. The common practice is to assume that all the dissolved CO2 is in the form of carbonic acid (H2CO3), which is produced in the reaction. What is the pH of a 0.0037 M solution of H2CO3? 18. Define base-dissociation constant 19. Calculate the concentration of OH- in a 0.15 M solution of NH3. 20. Define amines 21. A solution made by adding solid sodium hypochlorite (NaClO) to enough water to make 2.00 L of solution has a pH of 10.50. Using the information in Equation 16.37, calculate the number of moles of NaClO added to the water. 22. Calculate (a) Kb for the fluoride ion, (b) Ka for the ammonium ion. 23. By what factor does [H+] change for a pH change of (a) 2.00 units, (b) 0.50 units? 24. Complete the following table by calculating the missing entries and indicating whether the solution is acidic or basic. 25. The average pH of normal arterial blood is 7.40. At normal body temperature(37 °C), Kw = 2.4 * 10-14. Calculate[H+], [OH-], and pOH for blood at this temperature. 26. Addition of the indicator methyl orange to an unknown solution leads to a yellow color. The addition of bromthymol blue to the same solution also leads to a yellow color. (a) Is the solution acidic, neutral, or basic? (b) What is the range (in whole numbers) of possible pH values for the solution? (c) Is there another indicator you could use to narrow the range of possible pH values for the solution? 27. Is each of the following statements true or false? (a) All strong acids contain one or more H atoms. (b) A strong acid is a strong electrolyte. (c) A 1.0-M solution of a strong acid will have pH = 1.0. 28. Calculate the pH of each of the following strong acid solutions: (a) 8.5 * 10-3M HBr, (b) 1.52 g of HNO3 in 575 mL of solution, (c) 5.00 mL of 0.250 M HClO4 diluted to 50.0 mL, (d) a solution formed by mixing 10.0 mL of 0.100 M HBr with 20.0 mL of 0.200 M HCl. 29. Calculate 3OH-4 and pH for (a) 1.5 * 10-3M Sr(OH)2, (b) 2.250 g of LiOH in 250.0 mL of solution, (c) 1.00 mL of 0.175 M NaOH diluted to 2.00 L, (d) a solution formed by adding 5.00 mL of 0.105 M KOH to 15.0 mL of 9.5 * 10-2 M Ca(OH)2. 30. Calculate the concentration of an aqueous solution of NaOH that has a pH of 11.50. 31. Write the chemical equation and the Ka expression for the ionization of each of the following acids in aqueous solution. First show the reaction with H+(aq) as a product and then with the hydronium ion: (a) HBrO2, (b) C2H5COOH. 32. Lactic acid CH3CH(OH2COOH) has one acidic hydrogen. A 0.10 M solution of lactic acid has a pH of 2.44. Calculate Ka. 33. A 0.100 M solution of chloroacetic acid (ClCH2COOH) is 11.0% ionized. Using this information, calculate [ClCH2COO-],[H+], [ClCH2COOH], and Ka for chloroacetic acid. 34. A particular sample of vinegar has a pH of 2.90. If acetic acid is the only acid that vinegar contains (Ka = 1.8 * 10-5), calculate the concentration of acetic acid in the vinegar. the concentration of hydrofluoric acid. 35. The acid-dissociation constant for benzoic acid(C6H5COOH) is 6.3 * 10-5. Calculate the equilibrium concentrations of H3O+, C6H5COO-, and C6H5COOH in the solution if the initial concentration of C6H5COOH is 0.050 M. 36. Calculate the pH of each of the following solutions (Ka an Kb values are given in Appendix D): (a) 0.095 M propionic acid (C2H5COO), (b) 0.100 M hydrogen chromate ion(HCrO4-), (c) 0.120 M pyridine (C5H5N). 37. Saccharin, a sugar substitute, is a weak acid with pKa = 2.32 at 25 °C. It ionizes in aqueous solution as follows: What is the pH of a 0.10 M solution of this substance? 38. Calculate the percent ionization of hydrazoic acid (HN3) in solutions of each of the following concentrations (Ka is given in Appendix D): (a) 0.400 M, (b) 0.100 M, (c) 0.0400 M. 39. Citric acid, which is present in citrus fruits, is a triprotic acid Table 16.3). (a) Calculate the pH of a 0.040 M solution of citric acid. (b) Did you have to make any approximations or assumptions in completing your calculations? (c) Is the concentration of citrate ion (C6H5O7)-2 equal to, less than, or greater than the H+ ion concentration? 40. Consider the base hydroxylamine, NH2OH. (a) What is th conjugate acid of hydroxylamine? (b) When it acts as a base, which atom in hydroxylamine accepts a proton? (c) There are two atoms in hydroxylamine that have nonbonding electron pairs that could act as proton acceptors. Use Lewis structures and formal charges (Section 8.5) to rationalize why one of these two atoms is a much better proton acceptor than the other. 41. Write the chemical equation and the Kb expression for the reaction of each of the following bases with water: (a) dimethylamine, (CH3)2NH; (b) carbonate ion, CO32-; (c) formate ion, CHO2-. 42. Calculate the molar concentration of OH- in a 0.075 M solution of ethylamine (C2H5NH); Kb = 6.4 * 10-42. Calculate the pH of this solution. 43. Ephedrine, a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base: A 0.035 M solution of ephedrine has a pH of 11.33. (a) What are the equilibrium concentrations of C10H15ON, C10H15ONH+, and OH-? (b) Calculate Kb for ephedrine. 44. Although the acid-dissociation constant for phenol (C6H5O) is listed in Appendix D, the base-dissociation constant for the phenolate ion (C6H5O-) is not. (a) Explain why it is not necessary to list both Ka for phenol and Kb for the phenolate ion. (b) Calculate Kb for the phenolate ion. (c) Is the phenolate ion a weaker or stronger base than ammonia? 45. (a) Given that Ka for acetic acid is 1.8 * 10-5 and that for hypochlorous acid is 3.0 * 10-8, which is the stronger acid? (b) Which is the stronger base, the acetate ion or the hypochlorite ion? (c) Calculate Kb values for CH3COO- and ClO-. 46. Using data from Appendix D, calculate [OH-] and pH for each of the following solutions: (a) 0.10 M NaBrO, (b) 0.080 M NaHS, (c) a mixture that is 0.10 M in NaNO2 and 0.20 M in Ca(NO2)2.





