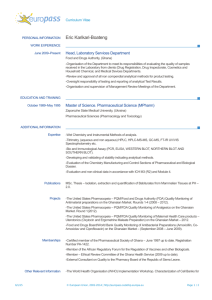
Global Health Supply Chain Program ($10.5 Billion)
advertisement
USAID Update 2014 Bureau for Global Health Christine Malati, PharmD Anthony Boni Lisa Ludeman, MSPH December 16, 2014 1 Outline • Structure of the Global Health Supply Chain Program • Updates on PQM and SIAPS 2 Global Health Supply Chain Program ($10.5 Billion) Single-Award Procurement & Technical Assistance IDIQ ($9.5b) With the Following Task Orders: 1. Family Planning 2. HIV/AIDS 3. Malaria 4. MCH HIV RTK Procurement IDIQ ($300m) Small Business Set Aside Multiple-Award Technical Assistance IDIQ ($500m) Up To Four Awards, Including one Small Business Set Aside Quality Assurance Contract ($123m) Business Intelligence and Analytics ($20m) GSA Contract Supply Chain Innovation & Research Annual Program Statement ($40m) # of Agreements TBD Single-Award Procurement & Technical Assistance IDIQ • 5-year single-award IDIQ – 4 task orders (HIV, malaria, family planning and reproductive health, maternal and child health) • Global Commodity Procurement and Logistics • Systems strengthening • Global collaboration 4 • • • • Five year single award IDIQ Procurement of RTKs Logistics Collaboration within the GHSCP and with other international stakeholders 5 Quality Assurance Award • Goal: Establish and implement a comprehensive QAP for USAID that provides global technical leadership regarding quality issues 1 Quality Assurance Program 2 Quality Control Strategy 3 Technical Leadership 4 Global Collaboration 6 Multiple Award Technical Assistance IDIQ • Goal: Improve the long-term availability of health commodities in public and private services • Objective 1: Systems Strengthening Technical Assistance – Strengthen incountry supply systems • Objective 2: Global Collaboration – strategic engagement to improve the longterm availability of health commodities 7 Multiple Supply Chain Assistance Cooperative Agreements and/or Grants Overall objective: Support research on a focused set of health supply chain systems and related commodity security issues in low and middle income countries. • Objective 1. Improve health supply chain systems through transformative changes that use industry best practice; • Objective 2. Improve the quantity, reliability, and efficiency of financing for health commodities and supply chain systems; • Objective 3. Improve governance and accountability for commodity security. 8 • $20m Contract • Purpose: to build and maintain as a Software as a Service (SaaS) USAID GH Business Intelligence and Analytics (BI&A) platform and provide technical services for and on behalf of USAID GH Bureau and the supply chain business initiatives. • Program Management • Data warehouse • Data mapping • Data migration • Knowledge Management Database 9 SIAPS: Recent Accomplishments • Advancing a framework and metrics to measure pharmaceutical systems strengthening • “Enhancing Health Outcomes for Chronic Diseases in Resource Limited Settings by Improving the Use of Medicines: The Role of Pharmaceutical Care” • Improving understanding of the vital role medicines play in Universal Health Coverage • Development of an accreditation framework for the establishment of national pharmaceutical continuous education programs in LMICs. • SIAPS West Africa Regional Project launches an Early Warning System (EWS) for HIV/AIDS commodities • Improving governance capacity for more transparent and accountable pharmaceutical systems • Applying knowledge management approaches to improve pharmaceutical management through collaboration with WHO PQM: Recent Accomplishments • Promoting the Quality of Medicines Program extended to September 2019 • Two PQM-supported manufacturers in China are the first to be prequalified for Capreomycin Active Pharmaceutical Ingredient (API). • PQM supported the first generic producer of Capreomycin to achieve WHO prequalification, thereby helping to reduce the cost of this medicine. • PQM supported the only major producer of Kanamycin to obtain GMP compliance status from the WHO Prequalification Program for the nonsterilized API of Kanamycin. • Ghana Food and Drug Authority QC lab receives ISO 17025 accreditation for compendial tests used to assess the quality of essential medicines. • With PQM support, the Ethiopian drug regulatory authority (FMHACA) laboratory became the first medical device laboratory in Africa to achieve ISO 17025 accreditation ((testing of male condoms). • Monograph developed (specifications, QC tests to perform, and methods) for Dihydroartemisinin-piperaquine phosphate tablets (DHAP), thereby enabling testing of this important FDC antimalarial. USAID Update 2014 Bureau for Global Health Christine Malati, PharmD Anthony Boni Lisa Ludeman, MSPH December 16, 2014 12