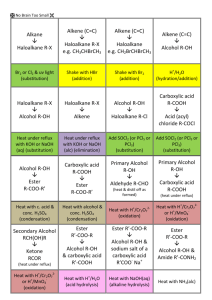
Calculations Alcohol Percentages
advertisement

Calculation Examples Alcohol Percentages Pg 150 • If you have 96% ethanol on your shelf you need to be able to dilute it doen to lower percentages of alcohol for making up potencies and dispensing. Any quantity of any percentage alcohol can be lade by applying the provided calculation: X desired volume multiplied by X desired% ethanol = Y volume on hand multiplied by Y % on hand Example 1 • You have 96% ethanol (R-OH) on your shelf • You want to make 200ml of 86% R-OH • You need to know how much distilled water you need to add to how much 96% R-OH to get 200ml of 86% • Xvolume = 200ml • X% = 86% • Y volume= ?? • Y% = 96% • Plug into the formula ………… Example 1 X desired volume multiplied by X desired% ethanol = Y volume on hand multiplied by Y % on hand 200ml x 86% 17200 17200 96% 179.166 = Yml x 96% = Yml x 96% = Yml = Yml This means that you need to measure out 179ml of 96% alcohol. You want a volume of 200ml of 86% so you need to add 200ml – 179ml = 21ml of distilled water to 179ml of 96% R-OH to get 200ml of 86% R-OH Example 2 • You have 86% ethanol (R-OH) on your shelf • You want to make 70ml of 20% R-OH • You need to know how much distilled water you need to add to how much 86% R-OH to get 70ml of 20% • Xvolume = 70ml • X% = 20% • Y volume = ?? • Y% = 86% • Plug into the formula ………… Example 2 X desired volume multiplied by X desired% ethanol = Y volume on hand multiplied by Y % on hand 70ml x 20% 1400 1400 86% 16.279 = Yml x 86% = Yml x 86% = Yml = Yml This means that you need to measure out 16ml of 86% alcohol. You want a volume of 70ml of 20% so you need to add 70ml – 16ml = 54ml of distilled water to 16ml of 86% R-OH to get 70ml of 20% R-OH