Principles of Biochemistry 4/e
advertisement
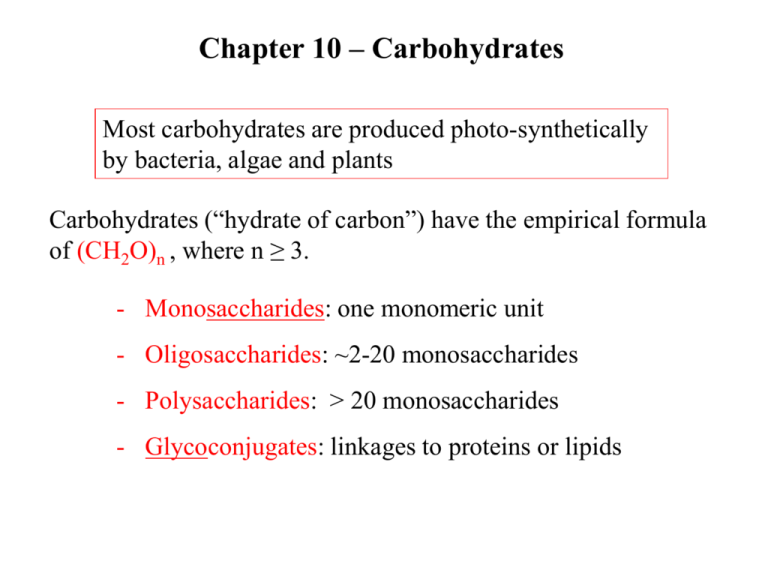
Chapter 10 – Carbohydrates Most carbohydrates are produced photo-synthetically by bacteria, algae and plants Carbohydrates (“hydrate of carbon”) have the empirical formula of (CH2O)n , where n ≥ 3. - Monosaccharides: one monomeric unit - Oligosaccharides: ~2-20 monosaccharides - Polysaccharides: > 20 monosaccharides - Glycoconjugates: linkages to proteins or lipids Saccharides of varying length Aldoses and Ketoses Trioses – three carbon sugars Ketotriose Aldotriose Saccharides of varying length Aldoses and Ketoses Tetroses – 4 carbon sugars Chiral designation comes from the most distant chiral carbon from the carbonyl D-sugars dominate in nature Saccharides of varying length Aldoses and Ketoses Figure 10.2 Pentoses – 5 carbon sugars Hexoses – 6 carbon sugars Chiral designation comes from the most distant chiral carbon from the carbonyl Figure 10.1 Isomeric forms of carbohydrates Epimers - Epimers – sugars that differ at only one of several chiral centers. example: D-Mannose is an epimer of D-Glucose Figure 10.4 Cyclization of D-fructose to form a- and bfructofuranose anomeric carbon Fig 10.3 Cyclization of D-glucose to form glucopyranose In aqueous solutions hexoses and pentoses will cyclize forming alpha (a) and beta (b) forms C1 is called the anomeric carbon In aqueous solutions, it is the ring structures that dominate These rings are NOT planar Figure 10.5 Figure 10.7 modified monosaccharides 6-deoxy-L-galactose Important in structural glycans Important in metabolic pathways Disaccharides and other Glycosides Glycosidic bond – the primary structural linkage in all polymers of monsaccharides Glycosides – glucose provides the anomeric carbon Figure 10.8 Structures of disaccharides: maltose More structures of disaccharides: lactose and sucrose Major carbohydrate in milk Most abundant sugar Sucrase, lactase and maltase are found on the outer surface of the epithelial cells lining the small intestine. Comes from the breakdown of starch and glycogen Reducing and Nonreducing ends of sugars - In linear polymeric chains of monosaccharides there is usually one reducing end (containing the free anomeric carbon) and one nonreducing end - Branched polysaccharides have a number of nonreducing ends, but only one reducing end Nonreducing end Reducing end (anomeric carbon) Read Clinical Insight pg 161 Polysaccharides - Homoglycans – homopolysaccharides containing only one type of monosaccharide. - Heteroglycans – heteropolysaccharides containing residues of more than one type of monosaccharide - The lengths and compositions of a polysaccharide may vary within a population of these molecules e.g.: starch and glycogen – storage polysaccharides cellulose and chitin – structural polysaccharides Starch - D-glucose is stored intracellularly in polymeric forms - plants and fungi store glucose as starch - Animals store glucose as glycogen - Starch is a mixture of amylose (unbranched) and amylopectin (branched every 25 sugars) (a) Amylose is a linear polymer containing only a-1,4-glycosidic bonds. (b) Amylopectin is a branched polymer also contains a-1,6-bonds Figure 10.12 Starch is stored by plants and used as fuel. Prentice Hall c2002 Chapter 8 16 a-amylase on amylopectin - a-amylase is found in plants and animals and is a hydrolase it is an endoglycosidase – hydrolyzes internal a-(14) glycosidic bonds Glycogen is is stored by animals and used as fuel. Prentice Hall c2002 Chapter 8 18 Glycogen - Glycogen is the main storage polysaccharide of humans - Glycogen is a polysaccharide of glucose residues connected by a-(14) linkages with a-(16) branches (one branch every 8 to 12 residues) - Glycogen is present in large amounts in liver and skeletal muscle Cellulose – a structural polysaccharide that is a Major component of cell walls of plants Cellulose has b-(14) glycosidic bonds Each glucose residue is rotated 180o relative to the next residue Extended hydrogen bonding between chains leads to bundles or fibrils Figure 10.14 Glycosidic bond Determines polysaccharide Structure. Bent structure Straight structure - Humans digest starch and glycogens ingested in their diet using amylases, enzymes that hydrolyze a-(14) glycosidic bond - Humans cannot hydrolyze b-(14) linkages of cellulose. Therefore cellulose is not a fuel source for humans. It is fiber. - Certain microorganisms have cellulases, enzymes that hydrolyze b-(14) linkages of cellulose. - cattle have these organisms in their rumen - termites have them in their intestinal tract Carbohydrates attached to proteins form glycoproteins Many glycoproteins are found as components of cell membranes (chapter 11) and take part in cell adhesion and binding. or Thr Mucins or mucoproteins are proteins which has Nacetylgalactosamine attached. This glycoprotein is found in mucus and is a lubricant. Two methods to anchor the protein to carbohydrates Figure 10.15 Glycosidic bonds between proteins and carbohydrates asparagine serine Thr is also used to make O-linkages Two methods to anchor the protein to carbohydrates Figure 10.16 N-linked oligosaccharides All N-linkages have a common core shown in grey. High mannose Protein residue Complex structure Protein residue Additional sugars can be attached to these cores to make diverse and unique structures Proteoglycans are glycoproteins were the protein is bound to a special class of polysaccharides called a glycosaminoglycan. This class of glycoprotein are used as structural components and lubricants. -In proteoglycans nearly 95% of the mass comes from the polysaccharide. -Proteoglycans function as lubricants, structural components in tissue and mediate the adhesion of cells to the extracellular matrix. Assignment Read Chapter 10 Read Chapter 11 Topics not covered: Section 10.4 Lectins