Powerpoint - UCSF Immunology Program
advertisement

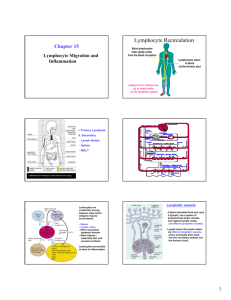
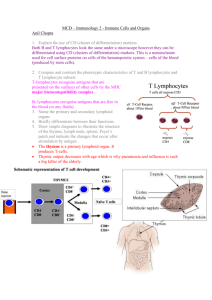
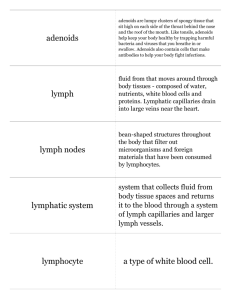
Homing and Inflammation Micro 204. Molecular and Cellular Immunology Lecturer: Jason Cyster jason.cyster@ucsf.edu • How do cells migrate from blood into tissue? – the four step model – role of selectins, chemokines and integrins • What controls recruitment of appropriate cell types (neutrophils, monocytes, lymphocytes) to the site of inflammation? • How do cells exit from tissue into circulation? • Are homing defects involved in human disease? Lymphocyte Homing • Constitutive trafficking of naive T and B lymphocytes to secondary lymphoid organs • in lymph nodes, Peyer’s patches, tonsil this requires active migration across blood vessels • Entry into secondary lymphoid organs is highly selective for lymphocytes • Egress from lymphoid organs involves distinct molecular mechanisms from entry Lymphocyte homing and recirculation (PP) Adapted from Anderson, artnscience.us Inflammation • involves local release of cytokines and chemokines by tissue cells in response to pathogen products or damage • cytokines cause increase in vascular permeability leading to local swelling, increased entry of antibody, complement, etc. • cytokines cause increased expression of adhesion molecules on vascular endothelium and these work together with chemokines to recruit cells - neutrophils, monocytes, NK cells and, later, effector lymphocytes The cascade (multistep) model of leukocyte extravasation Selectins are calcium-dependent (C-type) lectins (carbohydrate binding proteins) • L-selectin - entry to LNs, PPs – on lymphocytes (neutrophils) – binds specialized sulfated mucins (‘peripheral node addressins’ or PNAd) made by high endothelial venules (HEV) • P-selectin - early role in entry to site of inflammation – in Weibel-Palade bodies in endothelial cells and a-granules of platelets – translocates to membrane in response to thrombin, histamine, C5a, etc – binds PSGL-1, a tyrosine sulfated mucin present on neutrophils, some effector T cells • E-selectin - delayed role in entry to site of inflammation – cytokine inducible on endothelial cells (especially cutaneous) – binds carbohydrate ligand (sialyl-Lex) on neutrophil and effector T cell glycoproteins /glycolipids Selectin ligands Lymphocytes traverse high endothelial venules (HEVs) to enter lymph nodes Follicle or B zone HEV T cell area (paracortex) LN section stained with: B220 (pan B cell marker) PNAd (‘peripheral node address’ – sulfated glycans recognized by mAb MECA79) Key property of selectins is fast binding kinetics • The rapid kon and koff of selectin–carbohydrate ligand interaction allows flowing leukocytes to tether and roll along endothelial cells under shear flow • Rolling slows down flowing leukocytes and places them in proximity to endothelial cells where chemokines are transported and expressed Leukocyte rolling immediately after mild trauma depends on P-selectin - PSGL1 interactions wild-type PSGL1-deficient - both movies are of cremaster muscle venules within 30 minutes of surgery (selectins induced by inflammatory trauma) PSGL-1 = P-selectin glycoprotein ligand-1 Yang et al. 2000, JEM 190, 1769 The cascade (multistep) model of leukocyte extravasation Chemokines >40, structurally related basic proteins of ~10kD Four families: C, CC, CXC, CX3C chemokine NH2 S-S receptor Ga Lymphoid chemokines – help direct the homeostatic trafficking of cells into and through peripheral lymphoid tissues (e.g. CCR7-CCL21 and CCL19; CXCR5-CXCL13) Inflammatory chemokines – help recruit cells to sites of inflammation (e.g. CXCR2 / IL-8; CCR2 / MCP1; CCR5 / MIP1a) Chemokine (IL8)-mediated firm adhesion of rolling neutrophils Lymphocyte adhesion to HEV depends on chemokines normal lymph node CCL21/CCL19-deficient lymph node Stein et al., (2000) J. Exp. Med. 191, 61 The cascade (multistep) model of leukocyte extravasation Questions / Experiments for group discussion 1. What mechanisms might allow chemokines to act locally on endothelium? 2. Does the chemokine have to be expressed by the endothelial cell? 3. Could other classes of chemoattractants work at this step? The Integrin Family integrins are dimers of a and chains Most bind extracellular matrix proteins (e.g. fibronectin, laminin) but some have transmembrane ligands Integrins involved in leukocyte attachment to endothelium Integrin aL2 Ligands ICAM-1, ICAM-2 (ICAM-3) (CD11a/CD18, LFA1) a41 a47 VCAM-1, MADCAM-1, FN MAdCAM-1, VCAM-1, FN ICAM1 and VCAM1 are constitutively expressed on lymph node HEV and are upregulated on inflammed endothelium MAdCAM-1 is expressed by HEV in mucosal lymphoid tissues Integrin Activation • Integrins can be in resting (low affinity for ligand) and active states • intracellular signals cause ‘inside-out’ signaling in the integrin, converting it from an inactive to an active state • chemokine signaling can cause ‘inside-out’ signaling and activate integrins • ligand binding can cause ‘outside-in’ signaling that also promotes formation of the active state switchblade-like activation Inside-out signaling occurs by separation of the integrin cytoplasmic domains Kim, Carman & Springer 2003 Science 301, 1720 Multistep Model of Lymphocyte Transmigration into LNs Girard JP, Moussion C, Förster R. Nat Rev Immunol. 2012 Diapedesis / transendothelial migration • The forward migration of leukocytes across endothelium • Integrins may be involved in migration from point of attachment to endothelial cell junctions • Additional molecules involved – e.g. monocyte transmigration involves Junctional Adhesion Molecules (JAMs) and PECAM-1 (CD31) • Signaling occurs into the endothelial cell • May be promoted by shear stress (via mechanoreceptors?) Questions / Experiments for group discussion 1. What mechanisms might allow chemokines to act locally on endothelium? 2. Does the chemokine have to be expressed by the endothelial cell? 3. Could other classes of chemoattractants work at this step? 4. Do leukocytes only cross endothelium at cell-cell junctions? Questions / Experiments for group discussion 1. What mechanisms might allow chemokines to act locally on endothelium? 2. Does the chemokine have to be expressed by the endothelial cell? 3. Could other classes of chemoattractants work at this step? 4. Do leukocytes only cross endothelium at cell-cell junctions? 5. What mechanism might underly tissue-specific homing of lymphocytes? And other cell types? Homing specificity provided by the selectin/chemokine/integrin code Tissue selective chemokine and adhesion molecule expression in the organization of the immune system Kunkel & Butcher (2002) Immunity 16, 1 • Activated components of complement (C5a, C3a) also act as chemoattractants • Lipid Mediators (Prostaglandins & leukotrienes) can function as chemoattractants Abbas Fig. 2-16 INFLAMED TISSUE • Leukocyte adhesion deficiency (LAD) type I: – defects in 2 integrin -> defective neutrophil migration to inflammed skin, peritoneum; lymphocytes less affected due to continued use of a41, a47 – LAD patients have recurrent bacterial infections Experiment: You have a mutant mouse with symptoms of LAD but its integrins are intact. What other classes of molecules might you test as being potentially defective? How do cells exit from lymphoid organs? Diagram courtesy of Ted Yednock S1P MEDULLARY SINUSES -Lymphocytes express a receptor (S1PR1) for S1P - Egress involves migrating to S1P that is high in blood/lymph and low in the tissue Sphingosine 1-phosphate (S1P) Sph kinases Sphingosine S1P S1P lyase Lipid phosphatases S1PR1 (Edg1) S1PR2 S1PR3 Ethanolamine + Hexadecanal S1PR4 S1PR5 • Abundant in plasma (~1uM) and lymph (~0.1uM) • Made intracellulary by all cell types during sphingolipid degradation but only secreted by some cell types • Ligand for a family of G-protein coupled receptors (S1PR1-5) • S1P receptors have roles in blood vessel and heart development Lymphocytes express S1PR1 and exit lymphoid organs in response to S1P thymus, spleen lymph node, Peyer’s patch S1PR1 S1PR1 S1P lyase radiation resistant cells RBC S1P lyase radiation Lymphatic resistant Endothelial cells cells S1P blood S1P lymph • S1P is abundant in blood and lymph • RBC major source of blood S1P • Lymphatic endothelial cells are source of lymph S1P • Extracellular S1P is kept low inside lymphoid tissue • S1PR1 is a point of egress control Multi-step model of T cell egress 1. Cortical sinus probing 1. S1PR1 dependent entry 3. Capture in a region of flow and exit from lymph node T cells extend processes into cortical sinuses during egress decision-making Grigorova et al. 2009 Nature Immunol. 10, 58 Grigorova et al. 2009 Nature Immunol. 10, 58 Grigorova et al. 2009 Nature Immunol. 10, 58 Innate Immune stimuli cause a generalized lymph node egress shutdown Lymph Node Infection (e.g. dsRNA) 6h IFNa Non-specific Ag-specific Lymphocytes 12 h Model to account for IFNa-mediated block in egress Innate Immune Stimuli IFNa etc. S1P IFNa receptor CD69 S1P1 Internalize CD69 Egress promoting signal Egress Blocked CD69 gates migration of activated lymphocytes into cortical sinuses and egress from LN Grigorova et al., 2014 PNAS 107, 20447 Prolonged retention of antigenactivated lymphocytes S1PR1 • Antigen activated T cells downregulate S1PR1 mRNA and are retained Naïve • Recovery of S1PR1 after ~4 cell divisions associated with egress > Retention may help ensure appropriate clonal expansion and receipt of instruction signals before exiting • Activated B cells downregulate S1PR1 • A subset of plasma cells upregulate S1PR1 and exit spleen, LN to travel to BM Activated Effector Translational Impact of Homing Research • a41 blocking antibody (Natalizumab/Tysabri®) in use for treatment of Multiple Sclerosis and Crohn’s disease a47 blocking antibody (Vedolizumab/Entyvio®) approved May 2014 for ulcerative colitis and Crohn’s disease • CCR9 antagonist in phase III trial for Crohn’s disease • FTY720 (Fingolimod) first oral treatment for relapsing/remitting multiple sclerosis; in trial for ulcerative colitis • Inhibitors of prostaglandin and leukotriene synthesis have mutliple anti-inflammatory effects, including antagonizing cell recruitment • CXCR4 antagonists used to mobilize HSC from bone marrow • Led to advanced understanding of causes of Leukocyte Adhesion Deficiency (LAD) Recommended reading: • • • • • • • • • • • Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012. 12:762-73 Cyster JG, Schwab SR. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol. 2012. 30:69-94. Sperandio M, Gleissner CA, Ley K. Glycosylation in immune cell trafficking. Immunol Rev. 2009 230:97-113 Muller WA. Mechanisms of transendothelial migration of leukocytes. Circ Res. 2009 105:22330 Carman CV, Springer TA. Trans-cellular migration: cell-cell contacts get intimate. Curr Opin Cell Biol. 2008 20:533-40 Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007 317:666-70. Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nat Immunol. 2005 6:497-506. von Andrian, U. H. and Mempel, T. R. 2003. Homing and cellular traffic in lymph nodes. Nat. Rev. Immunol. 3, 867 Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr Opin Cell Biol. 2003 15:531-8 Carman CV, Springer TA. Integrin avidity regulation: are changes in affinity and conformation underemphasized? Curr Opin Cell Biol. 2003 Kunkel & Butcher (2002) Chemokines and the tissue-specific migration of lymphocytes. Immunity 16, 1