lab 9 (gases)
advertisement

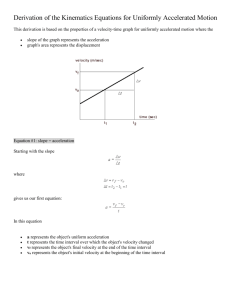
Ross Rybakowicz Lauren Roy Justin Pratt, Professor Jessa CHM 144 A November 24th 2014 Exploration of Different Gas Relationships Abstract The three experiments that were observed in this lab in order to understand gas laws were: Boyle’s Law, Gay-Lussac’s Law, and Charles’ Law. Boyle’s Law was determined by adjusting the volume of an enclosed gas sample and recorded the following pressure, all while the temperature was held constant. Gay-Lussac’s Law was studied by placing an enclosed gas sample into various baths with different temperatures. The pressure was then recorded at each point all while volume was being held constant. Charles’ Law was observed changing the temperature of a closed gas sample then recording the new volume that resulted, all while keeping the pressure. By completing these three experiment, it was determined that the relationship between volume and pressure was inverse, the relationship between pressure and temperature was direct, and the relationship between volume and temperature was direct. Introduction In order for the human population to survive on earth, gases are needed. Our atmosphere is full of vital gases such as Oxygen (O2), Carbon dioxide (CO2), Nitrogen (N2) etc., without gases, we could not survive. It is crucial to understand this form of matter, as it is so important to our daily life. There are four variables that gases possess to help develop the knowledge of their physical attributes. The four variables are pressure, volume, temperature, and the number of moles in the specific substance. These four variables then combine to form the ideal gas law, which is described as PV=nRT; pressure being P, volume being V, the number of moles being n, the gas constant being R, and temperature being T. This equation provides information on a gas’ ideal state of existence. The standard conditions of a gas would be at one atmosphere (atm) of pressure, 22.4L of volume, one mole of the specific gas, the temperature being 273.15K, and 𝑎𝑡𝑚 × 𝐿 the gas constant always being equal to 0.0821 𝑚𝑜𝑙 × 𝑘. Relationships of variables are able to be experimentally determined. By isolating two variables while holding the other two constant, the relationship between them can be calculated 1 by altering one of them and seeing how the other is affected. In this experiment, two variables were tested at a time holding moles of gas, due to the enclosed air sample, and the third variable constant. One of the variables being tested was manipulated and the other was then observed to experimentally determine the relationship between the two. Before this experiment occurred, three hypotheses were formed in order to predict the outcomes of the relationships of gases. For the first experiment, the relationship between pressure and volume, or Boyle’s Law, was observed. The hypothesis was that pressure and volume were inversely related meaning that as pressure increased, volume decreased, or vice versa. Boyle’s Law relates to the equation: P1V1 = P2V2. This was derived from P= 𝐧𝐑𝐓 𝐕 , since the gas constant is always the same, temperature, and number of moles were held constant, this equation was then able to be formed. Pressure and Volume are inversely related in this experiment meaning that what happened to the first variable, the opposite happened to the other. In order to test this, a syringe was used to change the amount of volume in the container, thus affecting the amount of pressure at a constant temperature. For the second experiment, Gay-Lussac’s Law, or the relationship between pressure and temperature were examined. The hypothesis for this relationship was that pressure and temperature are directly related meaning that as the pressure increased or decreased, the temperature did as well. Gay-Lussac’s Law refers to the equation P1/T1 = P2/T2, this again was derived from the equation P= 𝐧𝐑𝐓 𝐕 . Since the gas constant is always the same, volume and the number of moles is held constant, the equation was able to be determined experimentally. To test this relationship, an enclosed gas sample was placed into a beaker of water with varying temperature; the pressure was documented due to the change in temperature. The third and final experiment looked at Charles’ Law, or the relationship between volume and temperature. The hypothesis for this relationship was that temperature and volume are directly related, meaning that as one variable increased or decreased, the other did the same. Charles’ Law referred to the relationship V1T1 = V2T2, which again was derived from the ideal gas law PV=nRT; since the gas constant was the same, the number of moles and pressure were held constant, the relationship was able to be experimentally determined. To test this, an enclosed gas sample was submerged in a cooler of varying water temperatures, the pressure was allowed to equalize and the new volume was then recorded, thus determining the relationship. 2 Methods Experiment I: Relationship between Pressure and Volume (Boyle’s Law) An apparatus to test this relationship was constructed with a ring stand, a clamp handle, clamp foot, clamp a syringe holder, plunger, pressure sensor, and a 60 mL syringe. The temperature was held constant at room temperature. To collect the data, a LabQuest was used to track the pressure inside the enclosed sample. The syringe was initially placed at 55mL. A table was set up in the lab manual to record our observations. The volume was decreased in 5mL increments by tightening the handle to push down the plunger. For each new volume the pressure was recorded. Seven individual measurements were noted and used for calculations. Experiment 1 Calculations LoggerPro was used to determine this relationship of pressure and volume. Pressure, being the dependent variable, was plotted on the Y-axis while volume, being the independent variable, was plotted on the X-axis. A positive linear slope meant that the two variables were directly related while a negative slope with a curve meant that the two variables inversely related. Once the correct n value was determined and a linear slope was determined, the linear fit was analyzed displayed the equation of the line in P=m(V)n format. The proportionality constant, k=P/(V)n was calculated for each of the data pairs acquired throughout the experimental trials. The average of the k (proportionality constant) values was equivalent to m (the slope of the line). The standard deviation and the confidence interval at 95% were calculated as well. Experiment II: Relationship between Pressure and Temperature (Gay-Lussac’s Law) To test this relationship, an apparatus was used with a LabQuest device, temperature probe, pressure sensor, breaker, and a 25mL flask. The 25mL flask was sealed with a rubber stopper to assure the constant value of moles in the sample, which was then attached to a pressure sensor. The initial bath that the sample was placed in was 273K, which was made by cold water and crushed ice provided in the laboratory. Four additional baths of temperatures increasing in 10K increments were created for the sample of gas in which to be placed, making sure the temperature of the entire experiment varied between 40-50K. Each time the sample was submerged completely into the bath, the pressure was recorded and writing down in a lab manual. 3 Experiment II Calculations A graph was established in LoggerPro, displaying pressure, the dependent variable, on the Y-axis, and temperature, the independent variable, on the X-axis. Once the correct n value was determined, the linear fit was calculated showing the equation in P=m(T)n format. The proportionality constant was found for each individual pair of data points using k=P/(T)n. The average k value was equivalent to the m value in equation of the line. The standard deviation and confidence interval at 95% were also calculated. Experiment III: Relationship between Volume and Temperature (Charles’ Law) In this experiment, an apparatus was put together with a temperature probe, clamp, ring stand, a cooler, pressure sensor, bottle, stopper, stopcock, syringe, a ring stand and the LabQuest. An ice bath was made in the cooler for the initial trial. The utility clamp was used to place the temperature probe at the top of the cooler ensuring that the enclosed gas sample was fully submerged in the bath. Once the gas sample was submerged, the pressure was allowed to stabilize, which was recorded in the lab manual as the initial pressure. The volume was set to read 0.0mL. After the initial readings were documented, four additional baths with temperature increases of 10K, making sure there was a range between 40-50K, the syringe with the gas sample was submerged. The plunger was then pushed down until the pressure was the same as the initial pressure. A table was set up in the lab manual documenting each data pair of temperature and volume of the gas. Vbottle was acquired by filling up the bottle completely with water, then pouring the contents of the bottle into a graduated cylinder and recording the volume. Experiment III Calculations The total volume used in this experiment (Vtotal) was found by the equation: Vtotal = Vbottle + Vsyringe + Vtubing A graph was created on LoggerPro placing Vtotal, the dependent variable on the Y-axis, and temperature, the independent variable, on the X-axis. The correct n value was found, following by the equation of the line in Vtotal=m(T)n format using the linear fit. The proportionality constant for each set of data points was found using the equation k=Vtotal/(T)n. The average of the k values was equivalent to the m value in the equation of the line. The standard deviation and confidence interval at the 95% level were also calculated. 4 Results Experiment I: Relationship between Pressure and Volume (Boyle’s Law) Table 1-1 For Graph 1 Volume .055 .050 .045 .040 .035 .030 .025 (L) Pressure .9285 1.0090 1.1028 1.2270 1.3915 1.6147 1.8925 (atm) Table 1-1 displays each volume and pressure data point for each specific trial from the first experiment. Graph 1. Pressure and Volume Relationship Graph 1 shows the relationship between pressure and volume while the temperature was held constant. This was first a curve with a negative slope, which indicated that it was inversely related. The graph had to be manipulated by making the n value (-1) in order to get the correct linear fit and equation of the line. The correlation of the line was .9998, the Y-intercept was .1169, and the m value (slope) of the line was .04457. This was checked using the derived equation from the data points P= .04457(V)-1. 5 Table 1-2, Calculations for Experiment 1 Trial 1 2 3 4 5 6 7 k-value .0511 .0505 .0496 .0491 .0487 .0484 .0473 Average k-value: Standard Deviation: Confidence Interval at 95%: 𝒂𝒕𝒎 .0492 𝑳 1.3×10-3 .0492 ± 1.2×10-3 Table 1-2 shows each k-value, average k-value, standard deviation, and confidence interval at 95% calculated. These calculations depict the average k-value, standard deviation, and confidence interval at 95%. The m value (slope) was determined from the graph being .04457 and from the average kvalue being .0492. The m value from the graph does not fall into the range of appropriate kvalues (.0504-.0480) at the 95% confidence interval. It would fit if the confidence interval was changed to a lower percentage. Experiment II: Relationship between Pressure and Temperature (Gay-Lussac’s Law) Table 2-1 for Graph 2 Pressure .9285 .9685 .9923 1.0240 1.0533 (atm) Temperature 276.5 287.8 296.2 306.9 318.2 (K) Table 2-1 shows each data point that was recorded from each specific trial in the second experiment. 6 Graph 2. Pressure and Temperature Relationship Graph 2 illustrates the relationship between Temperature and Pressure while volume was held constant. When this graph was first plotted, the point made a straight line with a positive slope indicated the two variables were directly related. The n value was determined to be (1) since they were directly related. The correlation of this graph was .9982, the Y-intercept was .1091, and the m value was 2.976 × 10-3. This was checked with the derived equation being P=.002976(T). Table 2-2 Calculations Trial k-value 1 .003358 Average k-value: 2 .003365 3 .003350 4 .003337 .003344 Standard Deviation: 2.2 ×10-5 Confidence Interval at 95% level: .003344 ± 2.7 × 10-5 5 .003310 Table 2-2 shows the calculated values for each k-value, the average k-value, standard deviation, and confidence interval at 95%. These calculations show the k-values, the average of those values, the standard deviation, and the confidence interval at 95%. The m value was determined on the graph to be .002976 and through 7 the average k-value, which was .003344. The m value from the graph does not fall into the appropriate range (.003371-.003317) of the 95% confidence interval. If the percentage were to be lowered then the slope might fit. Experiment 3: Relationship between Temperature and Volume Table 3-1 for Graph 3 Volumetotal .0759 .0767 .0789 (L) Temperature 279.0 290.2 300.9 (K) Table 3-1 shows each individual volume and temperature data point .0809 .0837 311.4 321.4 Graph 3 Volume and Temperature Graph 3 illustrates the relationship between temperature and volume using the data points from experiment 3. Graph 3 is the relationship between temperature and volume while pressure was held constant. When this was first graphed, it had a positive linear slope indicating a direct relationship meaning as one of the variables increased or decreased, the other variable did the same. The correlation was .9818, the Y-intercept was .02329, and the m value (slope of the line) was 1.861 ×10-4. 8 Table 3-2 Calculations Trial 1 2 3 4 5 k-value 2.72 ×10-4 2.64 ×10-4 2.62 ×10-4 2.60 ×10-4 2.60 ×10-4 Average k-value: 2.64 ×10-4 Standard Deviation: 5.0 ×10-6 Confidence Interval at 95%: 2.64 ×10-4 ± 6.1 ×10-6 Table 3-2 shows each calculated k-value, the average k-value, the standard deviation, and the confidence interval at 95%. This table shows all of the calculated values for Experiment 3. The m value was determined from the graph as 1.861 ×10-4, however the average k-value was determined to be 2.64 ×10-4. The m value from the graph does not fit into the range of appropriate numbers from the 95% confidence interval, which were 2.70 ×10-4 to 2.58 ×10-4. If the percentage for the confidence interval were lowered then the m value from the graph would have fit. Discussion For the first experiment regarding pressure and volume, the hypothesis was that these two variables are inversely related meaning that as one variable increased or decreased, the other did the opposite. This hypothesis, known as Boyle’s Law, was supported due to the calculations. The experiment displayed correct results to show the inverse relationship. A negative curved slope was formed on the pressure and volume initial graph and the n value was determined to be (-1). The graph was then flipped to create a proper linear graph to attain the equation of the line. The second experiment pertained to pressure and temperature; the hypothesis was that these two variables were directly related meaning that if one increased or decreased, the other did the same. This hypothesis, derived from Gay-Lussac’s Law, was support from multiple calculations from the experiment. The data points collected created a positive line graph the first time it was plotted indicating a direct relationship. The n value was determined to be (1) since the graph was already linear. The third experiment was observing the relationship between volume and temperature; the hypothesis for this was that these two variables are directly related. This hypothesis was derived from Charles’ Law, was supported by the data collected and the results achieved. Once the data points were graphed, it displayed a straight line with a positive slope indicating a direct 9 relationship. Therefore, the n value was determined to be (1) since it was initially a positive linear graph. There were numerous areas in this experiment where an error could have occurred and skewed the data that was collected. A great source of error could have been the change in temperature for experiments two and three. Since the temperature of the water needed to stabilize, the temperature could have been recorded before the actual temperature was reached. Since the room temperature was much different than the water in the cooler that the experiments were been done in, the water was either always cooling down or heating up slightly. If this incorrect temperature were taken down, it would create a chain reaction of incorrect data, throwing off the other variable, which would then cause an inaccurate graph to be created. Since the graph would have been inaccurate, the line of best would be created incorrectly leading to very inaccurate calculations. Since the temperature was supposed to differ 10K each trial in those two experiments and there was suppose to be an overall difference of 40-50K, the change in temperature could greatly affect the ending results. Another outlet of error could be in the third experiment when observing Charles’ Law. In this experiment, the plunger was required to be adjusted to maintain the original pressure. If the original pressure were not reached in the specific trials then data would be incorrect. If the pressure for this experiment was not held constant, then the data would be inaccurate, potentially causing the experiment to not support Charles’ Law. As well as the other experiments, if one of the variables was not help constant that was suppose to be, the data would be skewed due to a third variable altering the effect of one of the variables being tested on the other. Conclusion This lab has given me a large spectrum of information. From the beginning of this lab, we were asked to create hypotheses about the three experiments that described the relationships between two variables. This taught me the skill of inferring because I had to predict the relationships between the variables based on the equation PV=nRT. The lab manual came in handy when I had to create these hypotheses and it made me feel much more confident in my abilities to predict and infer. This also provided an incentive to accomplish the lab accurately in order to see if my hypotheses were correct. Another major insight of information was the three gas laws, Charles’ Law, Gay-Lussac’s Law, and Boyle’s Law. Rather than just reading about these laws and concepts, I was able to test 10 them experimentally and accumulate my own understandings of how they worked. Experiencing a direct and inversely related relationship, in my opinion, is much better than reading about them in a lab manual. Watching three variables change and seeing how they affected the other variable in the experiment helped me understand the relationships very effectively. References Novak, Michael. "Chapter 11." General Chemistry Laboratory Manual. By Yasmin Jessa. N.p.: n.p., n.d. 127-36. Print. 11