isotopes
advertisement

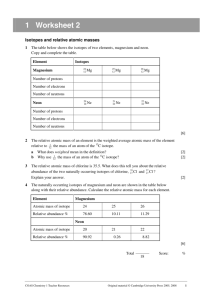
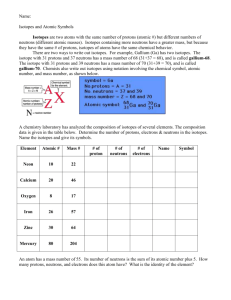
Isotopes Essential Question: How do atoms of the same element differ? What is an Isotope? • Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. • All elements consist of naturally occurring isotopes and artificially produced isotopes How are isotopes of an element similar? How are they different? The isotopes of an element have: • Identical Chemical Properties (this is because they have the same numbers of protons and electrons and subatomic particles are responsible for chemical behavior) • Different Physical Properties (different mass and different number of neutrons) How do we represent specific isotopes? • Nuclear symbols are used to represent specific isotopes • To write a nuclear symbol: • • • 1.) the symbol of the element is written first 2.) the mass number is written as a superscript to the left of the symbol 3.) the atomic number is written as a subscript to the left. Study the illustration below mass# atomic# X Li-7 isotope 7 3 Li Learning Check • Given a nuclear symbol, what does the top left number represent? – What does the bottom left number represent? Can we write isotopes in a different way? • You can also use the mass number and the name of the element to designate the atom or isotope – This is called hyphen notation • For example, two isotopes of carbon are carbon-12 and carbon-13 – The nuclear symbols for these two isotopes would be: 12 6 C 13 6 C Learning Check • What does the number after the hyphen represent? What do the numbers used when writing isotopes represent? • The mass number • total number of protons and neutrons in a specific nucleus of an atom. • The atomic number • always refers to the total number of protons in an atom. How do I figure out the number of neutrons when writing isotopes? • To determine the number of neutrons in a specific isotope – subtract the atomic number from the mass number • See the formula below Mass Number (neutrons + protons) Atomic Number (number of protons) (number of neutrons) Learning Check • Boron consists of two isotopes, B-10 and B-11 1.) Determine the number of neutrons in each of the isotopes a.) b.) B-10: B-11: 10 – 5 = 5 neutrons 11 – 5 = 6 neutrons 2.) Using the periodic table, write the nuclear symbol for each isotope given in hyphen notation 10 B 5 11 B 5 What does the atomic weight of an element depend on? • To identify isotopes the mass number is placed after the element’s name – Ex: chlorine-35 potassium-37 • The atomic weight of an element depends on the abundance of its isotopes. – If you know the mass of the isotopes and the percent (fractional) abundance of the isotopes, you can calculate the element's atomic weight. How do I calculate the atomic mass of an element based on percent natural abundance and isotopic masses? • Example: Chlorine has 2 naturally occurring isotopes Mass # Mass Percent Abundance 35 34.968852 75.77 37 36.965303 24.23 • To calculate the average atomic weight: – add the mass of each isotope multiplied by its percent abundance • This is the solution for chlorine: (34.968852) * (0.7577) + (36.965303) * (0.2423) = 35.45 amu Learning Check Nuclear Symbol Complete based on the following information: 29 protons, mass 65 Barium - 138 17 protons, mass 36 Tin - 120 16 protons, mass 30