File
advertisement

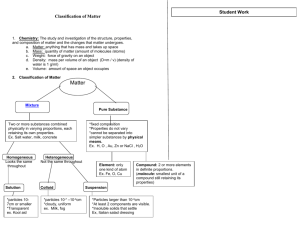
Unit 1 – Matter Classification of Matter Properties of Matter DO NOW What is Tyndall effect? Write three examples of an element. Write three examples of a compound. Agenda ‘Do Now’ Continue our discussion on matter A. Matter Flowchart MATTER yes MIXTURE yes Is the composition uniform? Homogeneous Mixture (solution) PURE SUBSTANCE no Heterogeneous Mixture Colloids no Can it be physically separated? yes Can it be chemically decomposed? Compound Suspensions no Element Pure Substances 1. Element composed of identical atoms EX: copper wire, aluminum foil Pure Substances 2. Compound composed of 2 or more elements in a fixed ratio properties differ from those of individual elements EX: table salt (NaCl) Another example.. Water What is the chemical formula for water? This has a 2:1 ratio It is an example of a pure substance AND also a compound. Mixtures Variable combination of 2 or more pure substances. Heterogeneous uneven distribution (suspensions & colloids) Homogeneous even distribution ( solutions) Orange Juice With pulp? Without pulp? Mixtures 1. Solution homogeneous very small particles no Tyndall effect particles don’t settle EX: rubbing alcohol Tyndall Effect Mixtures 2. Colloid heterogeneous medium-sized particles Tyndall effect particles don’t settle EX: milk The Tyndall Effect Colloids scatter light, making a beam visible. Solutions do not scatter light. Which glass contains a colloid? colloid solution Mixtures 3. Suspension heterogeneous large particles – can see Tyndall effect particles settle (needs to be shaken) EX: fresh-squeezed lemonade Mixtures Examples: jello colloid muddy water suspension Fog colloid saltwater solution Italian salad dressing suspension Mixtures vs. Compounds Components may be Components are in in any proportion Individual components retain their own identities Components may be separated physically When mixture is formed there is little to no evidence of a reaction fixed proportions Individual components lose their identities, new set of properties result Components may be separated only chemically When compound is formed there is evidence of a reaction Physical Separation Techniques Difference in Densities (density column – some objects float in others) Filtration (separate solids from liquids) Magnetism Chromatography Distillation (separation by boiling points) Evaporation (separate solids and liquids) Density Filtration Magnetism Evaporation Separation of a Mixture The constituents of the mixture retain their identity and may be separated by physical means. Separation of a Mixture The components of dyes such as ink may be separated by paper chromatography. PROBLEM This morning someone left me a ransom note in black ink and they stole Pickles. I have a few suspects, I need you all to help me find out who is responsible: Landlord: A Neighbor: B Mailman: C Ex-girlfriend: D Sister: E Separation of a Mixture Distillation Types of Properties Physical Chemical Properties that describe the substance itself, rather than describing how it can change Example: boiling point, color, size Properties that describe the substances ability to undergo changes that transform it into other substances Example: charcoal has the ability to burn in air Types of Physical Properties Extensive properties depend on the amount of matter that is present. Volume Mass Energy Content (think Calories!) Intensive properties do not depend on the amount of matter present. Melting point Boiling point Density Changes in Matter Physical Change Chemical Change Change in form or state of matter without altering chemical composition Examples: slicing a banana, boiling water, dissolving sugar Changing substance into new substance by reorganizing atoms…chemical bonds are made or broken Examples: burning, rusting, copper turns green 5 Indicators of a chemical change Color Change Light emitted (glow sticks, candle burning) Temperature change (happens on its own – you don’t supply heat) Precipitate forms (solid from 2 liquids) Gas production (you see bubbles) Three Phases Solids Definite shape/definite volume Molecules are tightly packed, but can still move slightly Most Dense state of matter (because particles are the closest) Liquids Definite volume/no definite shape (takes the shape of its container) Fluid – because it “flows” Particles are not as close as solids, but are more dense than gases Gases No definite shape or volume Least dense of the 3 states of matter because the particles are far apart Which state of matter are they? Phase Differences Solid – definite volume and shape; particles packed in fixed positions. Liquid – definite volume but indefinite shape; particles close together but not in fixed positions Gas – neither definite volume nor definite shape; particles are at great distances from one another Phase Changes Freezing (liquid to solid) Melting (solid to liquid) Evaporation (liquid to gas) Condensation (gas to liquid) Sublimation (solid to gas) Deposition (gas to solid) **** Phase changes are PHYSICAL changes!!!! Dry Ice What is dry ice? Process when you are making dry ice? Group up As a group, come up with example of these phase changes that occurs in your everyday life: Freezing Melting Condensation Evaporation