Selecting a buffer: - University of Illinois at Urbana
advertisement

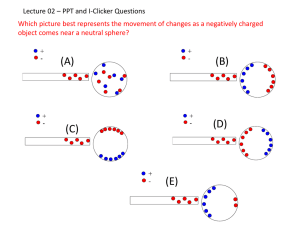
Some slides for thought – i-clicker activities Dr. Joaquin Rodriguez-Lopez University of Illinois at Urbana Champaign For Chem 222 Quantitative Chemical Analysis Lecture Updated May 2015 • I have found these slides to be very successful at nucleating discussion in groups of 2-3 students at the beginning of class. These slides are meant to introduce topics and to get students thinking about the different ways to see the same problem. Selecting a buffer: • We would like to prepare 100 ml of a pH =5.00 buffer. The available acids/salts in the lab are • A) acetic (pKa = 4.74) • B) benzoic (pKa = 4.18) • C) formic (pKa = 3.68) • D) pyridinium (pKa = 5.26) • i-clicker: Which should we use? • In what proportion of components? Note for thought: Notice that there are to “symmetric” answers to this problem – this question introduces the concept of buffer capacity … and Popcorn Take out paper, pencil and your i-clicker and let’s count. Do not look at your neighbor’s counts! How many kernels popped? A) Less than 170 D) 250-290 B) 170-210 E) +290 C) 210-250 Note for thought: This question introduces statistical error, distributions and sources of noise. The students are asked to count the number of pops in the actual pop-corn recording. The i-clicker response gives a histogram, from which the concept of standard deviation can be grasped. In the following slide, the intervals are made narrower. … and Popcorn Take out paper, pencil and your i-clicker and let’s count. Do not look at your neighbor’s counts! How many kernels popped? A) 195-215 D) 255-275 B) 215-235 E) 275-295 C) 235-255 Group discussion List all possible contributions to the “noise” in your measurement How would you refine your measurement? Do you think you can get rid of all sources of noise that could exist? Do you think using a better instrument is the best way to reduce noise? Note for thought: This is the same pop-corn example using a micro-phone and an oscilloscope i-clicker • At pH 13, all Mg2+ in a sample of hard water is precipitated as Mg(OH)2. 18.3 ml of 1.03 mM EDTA were required to titrate 20.5 ml of hard water, selectively chelating only the Ca2+ present. What is the Ca2+ concentration in the sample? A)0.92 mM B) 0.92 mM C) 0.92 M D)1.12 mM E) 1.45 mM Selectivity towards ions K+ Na+ Mg2+ Ca2+ Fe2+ Fe3+ log K = 0.8 log K = 1.86 log K = 8.79 log K = 10.65 log K = 14.30 log K = 25.1 Note for thought: Tests if student understands the procedure. Effectively, Mg has no role in the calculation Accuracy and Precision A B C D E If the center of the target represents the “accepted answer” then, 1) The response represents an accurate and precise measurement 2) The response is neither accurate nor precise We cannot “eyeball” our results all the time…What is a reasonable precision How do I calculate it? What contributes to different outcomes? For that we will use Statistics – Let’s warm up the numbers! 7 Simple, right? The Mars phoenix lander contained a microelectrode voltammetry system. The current (I, in Amperes) produced on the microelectrode due to the oxidation of analyte is: I = 4nFaDc Where: n is the transferred number of electrons, here n=1 F is Faraday’s constant: 96,485 C/mol, a is the radius of the electrode: 12.5 mm here D is the diffusion coefficient: 3.2×10-5 cm2/s c is the concentration of analyte: assume 2.512 mM And the answer is: A) 4 nA B) 0.39 mA C) 0.4 mA D) 39 nA E) 38.779 nA Note for thought: Tests the correct use of units and their conversion 8 Dynamic electrochemistry Activity cathodic Sequence for tetramethyl p-phenylenediamine anodic Region 1 Voltammogram started here Initial reagent Products Scan direction 1. General questions: TMPD can be reacted at an electrode, as shown in the voltammogram to the right a) Is TMPD being oxidized or reduced? Give two arguments for this 2. The voltammogram a) Focus on Region 1 – Identify: The limiting current The E0 of the reaction b) What does the combination of Region 1 and Region 2 tell you about the mechanism and electrons transferred in the reaction? Discuss your conclusion. Region 2 Potential vs. NHE / V c) For the limiting current: Ilim = 4nFaDc where n = # of electrons, F = 96,485 C/mol, a is the radius of the electrode (= 300 nm) and D = 1×10-9 m2/s … What is the concentration of TMPD in the solution? d) If the beaker has 10 ml of solution… how long would it take you to transform all TMPD at the rate set by the limiting current? i-clicker items • What is the concentration of TMPD ? • • • • • A) 26 M B) 26 mM C) 13 mM D) 26 nM E) Negligible Note for thought: Tests that students understand the quantitative nature of electrochemical measurements. i-clicker items • How long would it take to transform the contents of the beaker? • • • • • A) 2,650 years B) 83.9 x 109 seconds C) Cannot be determined with the info provided D) 15 picoseconds E) 1 million years Note for thought: micro-electrodes for practical purposes perform non-destructive analysis