Oaks: a 'worst case scenario for the biological species concept'1
advertisement

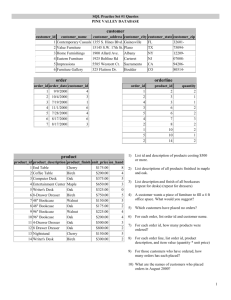
Who am I this time? The affinities and [mis]behaviors of Hill’s oak (Quercus ellipsoidalis) Andrew Hipp Jaime Weber Alka Srivastava The Morton Arboretum Oaks: a ‘worst case scenario for the biological species concept’1 1Jerry Coyne and H. Allen Orr “Hybridization and introgression in Quercus alba James W. Hardin (1975) J. Arnold Arboretum 56: 336–363 Distribution of cytoplasmic haplotypes by species Dumolin-Lapegue et al. (1997) Evolution 53: 1406-1413 Gonzalez-Rodriguez, A. et al. (2004) Morphological and RAPD analysis of hybridization between Quercus affinis and Q. laurina , two Mexican red oaks. American Journal of Botany 91:401-409. Quercus petraea – Sessile oak source: www.habitas.org.uk/flora/ Quercus robur – Pedunculate oak Source: http://www.bomengids.nl/ “Species status of hybridizing oaks” Muir et al. (2000) Nature 405:1016 Scotti-Saintagne et al. (2004) Genetics 168: 1615–1626. A potent issue in the Great Lakes region is whether the endemic Hill’s oak (Q. A second issue is how and to what extent these species are mixed up with black ellipsoidalis) and the Appalachian scarlet oak (Q. coccinea) represent two biological oak (Q. velutina). entities or just endpoints on a morphological continuum. Q. ellipsoidalis Q. velutina Q. coccinea Genetic (AFLP) data have previously been used to demonstrate that scarlet oak is cleanly separated from Hill’s oak and black oak, which are themselves largely distinguishable from one another, but less cleanly separated. NMDS on a Jaccard’s pairwise distance matrix, AFLP data. Final stress = 30.40305 Final instability = 0.01349 Number of iterations = 100 R2 = 0.216 (axis 1) + 0.287 (axis 2) = 0.503. MCMC clustering (STRUCTURE), admixture model, allele freq’s correlated, flat prior on id’s. Q. coccinea Q. ellipsoidalis Hipp and Weber (2008) Syst. Bot. 33: 148–158. Q. velutina Sampling for the current study: 851 individuals from six species – black oak, Hill’s oak, scarlet oak, pin oak, red oak, and Shumard’s oak – from 58 sites, including 803 individuals from the three focal species and 24 from red oak. Questions driving this study General questions (the ones biologists all have): • How does the ecological and morphological coherence we observe in oak species persist in the face of introgression? • What do oak species look like when we tease apart shared ancestry and ongoing gene flow? Particulars (questions that keep at least some IOS members awake at night, but probably not all biologists): • What are the genetic disjunctions among Hill’s oak, scarlet oak, and black oak? • What are the evolutionary (phylogenetic) relationships among black oak members of eastern North America? • What is the pattern of hybridization / local gene flow among Hill’s oak, scarlet oak, and black oak? The New World black oak group (Quercus section Lobatae) has proven particularly challenging in previous molecular work, and at the outset of our study, it wasn’t at all clear that we would be able to distinguish species from one another. Aldrich et al. (2003) Can J. For Res 33: 2228–2237 AFLP Methods: 1. Double digestion with MseI and EcoRI 2. Adapter ligation 3. Amplification with unlabelled 1-base selective primers 4. Amplification with 3-base selective primers, one of which is fluorescently labelled 5. Product is run out on ABI sequencers (capillary or slab) with internal lane standard For this project we screened 128 primer pairs and selected one. Each individual is scored as having (1) or not having (0) an allele at each position; that is, as having or not having a band of a particular size. Polyacrilimide gel showing AFLP banding patterns for two primer pairs (blue and yellow) with an internal lane standard (red) What kind of data do you get out of this process? Each ID indicates a single individual sampled in the field. Each number indicates the size of a genetic marker, an AFLP band that indicates the presence (1) or absence (0) of a specific gene copy at a specific region of the genome. 50 52 55 57 58 59 62 63 64 65 vel2406 1 1 1 1 0 0 0 1 1 1 rub2407 1 1 1 1 0 0 0 1 1 1 vel2416 1 1 1 1 0 1 0 1 1 1 vel2418 1 0 1 1 0 1 0 1 1 1 ell2422 1 1 1 1 0 0 0 1 1 1 ell2424 1 1 1 1 0 0 0 1 1 1 eXr2426 1 1 1 1 0 0 1 1 1 1 ell2431 1 1 1 1 0 0 0 1 1 1 ell2438 1 1 1 1 1 0 0 1 1 1 ell2439 1 1 1 1 0 0 0 1 1 1 ell2442 1 1 1 1 0 0 0 1 1 1 rub2447 1 1 1 1 0 0 0 1 0 1 eXv2450 1 1 1 1 0 0 0 1 1 1 These data are good for estimating genetic distances between individuals vel2406 vel2418 ell2422 ell2424 ell2431 ell2438 ell2439 ell2442 eXv2450 Interspecific D = 0.0503 ± 0.0011 Intraspecific D = 0.0476 ± 0.0021 vel2406 0.0000 vel2418 0.0485 0.0000 ell2422 0.0514 0.0467 0.0000 ell2424 0.0493 0.0474 0.0385 0.0000 ell2431 0.0517 0.0471 0.0389 0.0478 0.0000 ell2438 0.0455 0.0559 0.0527 0.0539 0.0657 0.0000 ell2439 0.0575 0.0489 0.0489 0.0305 0.0589 0.0457 0.0000 ell2442 0.0474 0.0544 0.0428 0.0462 0.0460 0.0548 0.0415 0.0000 eXv2450 0.0635 0.0646 0.0401 0.0465 0.0522 0.0458 0.0449 0.0508 0.0000 Methods • • • • Plants were collected from the three target species (Q. coccinea, Q. ellipsoidalis, Q. velutina) from populations throughout the Great Lakes region, along with examplars of co-occurring, related species (Q. palustris, Q. rubra, Q. shumardii). Individuals were classified to species using morphological and genetic methods and assigned to populations based on locality. AFLP data were collected for all individuals. Analyses: a. Ordination, ME trees, Bayesian clustering approach, and linear regression used to visualize patterns b. Permutation tests used to test the significance of gene flow relative to a null model of no gene flow. Scarlet oak: Quercus coccinea Scarlet oak, Chemung Co., NY Black oak, Chemung Co., NY Black oak, Taltree Arboretum Black oak, Taltree Arboretum Hill’s oak, Taltree Arboretum Black oak, Quercus velutina Hill’s oak, Quercus ellipsoidalis Finding 1: As we throw more data at the problem, it becomes clear that Q. coccinea and Q. ellipsoidalis are sister species, and populations of each species form discrete clusters. Minimum evolution (ME) tree of populations based on pairwise distance matrix of AFLP data (Nei’s unbiased D for populations) Dataset: 259 AFLPs (2 primer pairs) 718 ind’ls in 81 populations 80% genetic threshold Minimum evolution (ME) tree of populations based on pairwise distance matrix of AFLP data (Jaccard’s distance); nonparametric bootstraps above branches Dataset: 2228 AFLPs (10 primer pairs) This sister species relationship between Hill’s oak and scarlet oak is satisfying given the difficulty of distinguishing the two species in the northeastern Illinois / northwestern Indiana area. Quercus coccinea is limited to perhaps a single site in the region (Tinley Creek FP, Cook Co., IL). This result is also satisfying in light of the variety in acorn morphology of Q. ellipsoidalis, which is almost dazzling. “Though the extremes under which the acorns of Quercus ellipsoidalis occur pass into one another so that the recognition of forms based on them is scarcely more than a convenient way of ensuring their reference to this alliance, the fruit of a given tree is fairly uniform.... So far as I know, each of the fruit forms may be expected wherever the species occurs.” William Trelease, 1919 In a recent issue of the International Oak Journal, Dave Shepherd has published a long-overdue reinvestigation of morphological variation in Quercus ellipsoidalis and Q. coccinea. One of the many observations in this study is the morphological similarity between certain Q. coccinea populations of northeastern North America and Q. ellipsoidalis of the Great Lakes region. Shepard (2009) International Oak Journal 20 [Figure 4, Discriminate functions analysis]. Our findings on the relationships among populations within the species are consistent with Shepard’s morphological work, demonstrating a close genetic similarity between Q. ellipsoidalis and the Q. coccinea populations we sampled in New York state. It is still striking how broad the genetic disjuncture between these taxa is. Scarlet oak Black oak Nonmetric multidimensional scaling (NMDS) ordination of populations based on pairwise distance matrix of AFLP data (Nei’s unbiased D for populations) Dataset: 259 AFLPs (2 primer pairs) 728 ind’ls in 75 populations 65% genetic threshold Hill’s oak Finding 2: Analyzed at the individual (rather than population) level, the species separate out pretty cleanly, though with some remarkable misclassifications between black oak and Hill’s oak – i.e., incongruence between our identifications based on morphology and the population assignments based on genetic data. The incorporation of genes from one species into another is the definition of genetic introgression, and the presence of such individuals supports the hypothesis of gene flow between the two species. Finding 3: Of the three species, Quercus ellipsoidalis exhibits the greatest genetic variance (among-population). This might be a sampling bias – we sampled more Q. ellipsoidalis than other species – but our geographic range in Q. coccinea and Q. velutina was greater than in Q. ellipsoidalis, so the potential to detect regional genetic variants in those species was also good. NW IN Michigan We can correlate spatial data – pairwise geographic distances – with genetic data – pairwise genetic distances – to investigate whether this genetic variance has a geographic pattern. If there a positive correlation between geographic distance and genetic distance within a species, then we can conclude that genetic variance is spatially autocorrelated: populations that are near one another are also genetically similar, due either to gene flow or geographic dispersal patterns. We can also use spatial data to investigate whether sympatric species share alleles. We can detect localized gene flow between species either by comparing genetic similarity among paired populations of the focal species… … or by correlating pairwise geographic distances with pairwise genetic distances on among-species (rather than within-species) comparisons. A significant positive correlation tells us that the species are or were recently sharing genes locally. Finding 4: There appears to be allele sharing (gene flow) between Q. ellipsoidalis and Q. velutina in the upper Great Lakes region, where the two occur in sympatry. The effect persists even when only individuals with 95% assignment to one species are include in analysis. However, this effect is not very strong and bears further study. Pin oak – Q. palustris Hill’s oak – Q. ellipsoidalis Red oak – Q. rubra Black oak – Q. velutina Scarlet oak – Q. coccinea Jensen 1977. Taxon 26: 399—407. Conclusions 1. Hill’s oak and scarlet oak are sister species, and their morphological similarity is therefore a consequence of shared ancestry, not convergence or gene flow. Variance in the morphology of both species, such that they overlap morphologically, may be reflected in their population genetic structure. However, the strong genetic disjuncture between the two is consistent with recognition at the species level. 2. Within Hill’s oak, there is substantial geographically-structured genetic variation that is not present in the other two species. 3. There appears to be local allele-sharing as well as introgression between Hill’s oak and black oak; however, the effect is not strong and bears further study. Upshot: Oak species—even the worst of them—are genetically cohesive even in the face of ongoing local gene flow, and the nuclear genome bears the stamps of both phylogeny and introgression. Jaime Weber, Research Assistant Q. velutina 2521 Q. ellipsoidalis 2528 Q. ellipsoidalis 2532 Q. velutina 2544 Alka Srivastava, Volunteer Jason Sturner, Herbarium Assistant Acknowledgements Selected collaborators on this project: Jeannine Cavender-Bares, Paul Manos, Anton Reznicek. Field and Lab assistance: J. Sturner, K. Feldheim, E. Sackett-Hermann, L. Anderson, D. Bassett, G. Fewless, M. Bowles, R. Ewert, A. Gapinski, J. Hitz, C. Kirschbaum, D. Ladd, J. Mendelson, M. Nee, B. Olker, J. Wibbenmeyer, J. Yunger, the Forest Preserve Districts of Cook, Lake, DuPage Counties, Illinois, Albany Pine Bush Preserve, Indiana DNR, and numerous others. Funding: The Morton Arboretum, Michigan Botanical Club, and The American Philosophical Society. And thank you!