Water Potential & Osmosis Worksheet - AP Biology
advertisement

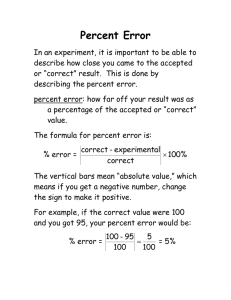
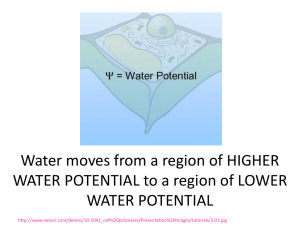
Thomases’ AP Biology Water Potential and Osmosis Worksheet Name: _________________________ Per: ________ 1. Write the formulas for water potential and solute potential and explain the meaning of each symbol. ψ = ψs - ψp ψs = solute potential = -iCRT ψp = pressure potential 2. Water potential is measured in what units? Bars (liter bars) (also Megapascals (mPa) 3. What two factors determine water potential? (See the equation you wrote). solute concentrate and pressure 4. Explain what direction water will move regarding water potential. high water potential to low water potential 5. Pure water has a water potential of_______0 bars____ 6. If water potential is -2bars and solute potential is -2bars, then pressure potential = ψ = ψs - ψp ___0 bars_____ 7. If solute potential is -3 bars and water potential is 0 bars, then the pressure potential ψ = ψs - ψp = ___3 bars____ 8. If water potential is -9 bars and pressure potential is +4 bars, then solute potential = ψ = ψs - ψp __-13 bars__ 9. Which beaker(s) contain(s) a solution that is hypertonic to the bag? 1, 3, 5 1 2 3 4 5 10. Which bag will gain the most mass after 30 minutes? Most: bag 4 will gain the most 11. If a cell has a ψ of -3 and the solution it is in has a ψ of -2, which way will the water move? into the cell 12. If the molar concentration of a sugar solution in an open beaker is 0.4M, calculate the solute potential at 27 degrees. Round to nearest hundredth. ψs = -iCRT ψs = (-1) (0.4M) (0.0831bar) (273+27) ψs =-9.972 bars 13. From the beakers below, in beaker B, what is the water potential of the distilled water in the beaker, and of the beet core? water 0 bars beet core = -0.2 bars ψp=0.2 ψs =-0.4 ψp=0.4 ψs =-0.4 14. Which of the following statements is true for the diagrams above? a. The beet core in beaker A is at equilibrium with the surrounding water b. The beet core in beaker B will lose water to the surrounding environment. c. The beet core in beaker B would be more turgid than the better core in beaker A d. The beet core in beaker A is likely to gain so much water that its cells will rupture e. The cells in beet core B are likely to undergo plasmolysis 15. Calculate the solute potential of a 0.15M salt solution in a beaker at 20o C. Round to nearest thousandth. ψs = -iCRT ψs =(-2)(.15M)(0.0831liter bars)(273+20 degrees) ψs =-7.304 bars 16 a-c. Given the following scenarios, draw an arrow representing the movement of water across these semipermeable membranes. a. b. c. ← ψ = 1.2 ψ = 3.2 → ψ=0 ψ = -2.3 ← ψ = -2.3 ψ=0 Calculate the water potential for the following molarities of sucrose given: ΨS = -I C R T. Ψ = ΨS + Ψp Sucrose’s ionization constant is 1 The pressure constant (R) is 0.0831 liter bars/mole ºK. The experiments were run at 23 ºC. (Convert to Kelvin by adding Celsius to 273). 17. 1.1 Mol/Liter = __________________ ΨS = -I C R T ΨS = -27.05736 bars 18. 0.3 Mol/liter = ___________________ ΨS = -I C R T ΨS= -7.37928 bars 19. 0.0 Mol/Liter = _______________0_bars___ 20. 7 Mol/Liter = _________-172.18 bars__________ 21. Describe the relationship between temperature to water potential. Higher temp means more molecular collisions so water potential goes up 20. Describe the relationship between tonicity to water potential. As the concentration of solute goes up, the water potential goes down because water locks around the solute in hydration spheres and is not free to do work.. Water will move from hypotonic (high water potential) to hypertonic (low water potential.)