File
advertisement

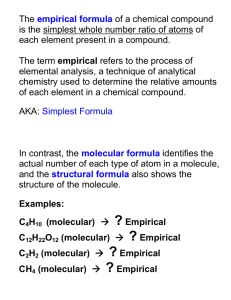
Calculations in Chemistry- part 2 Molar volume • • • • • • • • • What is the mass of: 600cm^3 of Ammonia gas NH3 at RTP? 0.43g 1000mL of Methane CH4 gas at RTP? 0.67g 4800 cm^3 Oxygen gas O2 at RTP? 6.40g 10000 cm^3 of Nitrogen dioxide NO2 gas at RTP? 19.17g Molar volume • • • • • • • • • What is the volume of: 0.003 moles of hydrogen H2 at RTP? 72cm^3 8g of oxygen O2 gas at RTP? 6000 cm^3 120g of sulphur dioxide SO2 gas at RTP? 45000 cm^3 5.6 g of Nitrogen N2 gas at RTP? 4800 cm^3 Reacting volumes • Gases react according to the mole ratio in a balanced equation. • Volume of a gas is proportional to the moles. • 2H2(g) + O2(g) 2H2O(g) • In the above reaction, hydrogen, oxygen and water vapour are in the ratio 2:1:2. • If 100 cm^3 of hydrogen were completely reacted, 50cm^3 oxygen are used and 100cm^3 water vapour are produced. • Nitrogen and Hydrogen react according to the equation. • N2(g) + 3H2(g) 2NH3(g) • If 150cm^3 of ammonia are formed, what will be the volume of nitrogen and hydrogen reacted? • N2:NH3 = 1:2 So nitrogen reacted is 75cm^3 • N2: H2 = 1:3 So hydrogen reacted is 225cm^3 • In the following reaction 200 cm^3 of Sulphur dioxide gas was mixed with 175cm^3 oxygen. • 2SO2(g) + O2(g) 2SO3(g) • (a) Balance the equation • (b) Which gas will be completely used up? • SO2 (limiting agent) • (c) What is the volume of SO3 produced? • 200cm^3 • (d) Name gases present when the reaction finished? • SO3 and remaining O2 • (e) What is the final volume of the gaseous mixture? • 200cm^3 (SO3)+ 75cm^3 (O2) = 275cm^3 • Methane burns in air according to the following equation: • CH4(g) + 2O2(g) CO2(g) + 2H2O(g) • In an experiment, 600cm^3 of methane were burnt in 1500cm^3 of O2. • (a) Which chemical is the limiting reagent? • Methane is the limiting agent • What is the volume of CO2 and H2O are produced? • CO2 = 600cm^3 H2O = 2 X 600 = 1200cm^3 • What volume of unreacted gas is left? • Unreacted gas is Oxygen = 1500 – 1200 = 300cm^3 • What is the final volume at the end of the experiment? • Final volume contains CO2, H2O and excess O2 • 600+ 1200+ 300 = 2100cm^3 % Yield IGCSE Chemistry • A student was asked the following calculation: • Copper metal is made from copper(II) oxide by heating it with carbon powder. The equation is as follows: • 2CuO + C 2Cu + CO2 • 8.0g of copper (II) oxide was heated with 4.0g of carbon powder. • (a) How many moles of copper(II) oxide was used? • (b) How many moles of carbon were mixed? • (c) What is the limiting reagent? • (d) What mass of copper is made in this calculation? • (e) How many g of the other reagent is also left at the end of the reaction? • (f) How many moles of CO2 were produced? • (g) What is the volume of this CO2 at room temperature and pressure? The student made calculations and made the following results. • • • • • • • • (a) moles of copper(II) oxide = 0.1 (b) moles of carbon mixed = 0.33 (c) Limiting agent is Copper(II) oxide (d) mass of copper made = 6.4g (e) g of other reagent(excess) left = 3.36g (f) Moles of CO2 produced = 0.05 (g) Volume of CO2 produced = 0.05 x 24000 = 1200 cm3 • The teacher asked the student about the expected amount of copper. • The student read the previous calculations and said 6.4g • The teacher then asked the student to conduct an experiment using 8.0g of copper(II) oxide and 4.0g of carbon. • The student conducted the experiment using the following set up • What is left in the test tube when the experiment is over? • What the student might have seen with lime water? Why? • What can be the reason the test tube is slanted slightly down? • The student separated copper produced from remaining carbon and weighed. She found that the weight is smaller than what she calculated. That is 4.8g • Why you think the amount of copper is smaller than what she calculated? • What you call the amount of product calculated and the amount of product really produced in an experiment? • If the formula to calculate % yield is: find out the percentage yield of copper. Try this question now: • Zinc displaces copper from copper(II) sulphate by displacement reaction according to the following equation: • CuSO4 + Zn ZnSO4 + Cu • In a reaction 3.2g of copper(II) sulphate was mixed with 3.0g of Zinc. • (a) How many moles of copper(II) sulphate are present in 3.2g? • 0.02 moles • (b) How many moles of Zinc is present in 3.0g? • 0.046 moles • What chemical is the limiting agent? • Copper(II) sulphate is the limiting agent • How many moles of the other chemical is left at the end of the reaction? • 0.046 – 0.020 = 0.026 moles of Zinc • What mass is it? • 0.026 x 65g = 1.69g • What mass of copper is produced? (theoretical yield) • 0.02 x 64g = 1.28 g • In an experiment, 1.10g of copper was produced (Experimental/actual yield). Find out the % yield of copper FORMULA IGCSE Chemistry Formula • Two important types of formulae in Chemistry are: • 1. Empirical formula This shows which elements are present in a compound and the ratio of various atoms in one molecule. E.g Empirical formula of Benzene is CH • 2. Molecular formula Molecular formula shows actual number of various atoms in a molecule. E.g Molecular formula of Benzene is C6H6 • When you multiply Empirical formula with a number, you get the molecular formula. • In the example of benzene, when you multiply Empirical formula with 6, you get the molecular formula • (CH) X 6 C6H6 • You can calculate the number if you know the empirical formula and molecular mass. • First find the empirical formula mass by adding various atomic masses. • Then divide the molecular mass with empirical formula mass, you get the number. Molecular formula • Molecular mass will be given in the question. • In the example of Benzene, molecular mass is given as 78 and empirical formula is CH • Find Empirical formula mass first = 12 + 1 = 13 • Divide molecular mass with empirical formula mass to get number (n) • 78/13 = 6 • Now multiply Empirical formula with 6 you get molecular formula • (CH) X 6 = C6H6 How to find the empirical formula? • To find the empirical formula, you need the mass (g) or (%) of different element. • Step 1:convert mass (%) in to moles by dividing with molar mass of the element. • Step 2:Find the mole ratio by dividing each moles calculated in step one with the smallest mole. • Step 3: Round off the moles to the nearest whole number and write the empirical formula. Example • A hydrocarbon contains 92.3% C and 7.7% H. Its molecular mass is 78. find the empirical formula and molecular formula of the compound. • Empirical formula Empirical formula = CH • Now find out n • 78/13 = 6 • Molecular formula is (CH) X 6 • C6H6 Another example • Determine the empirical formula of the compound containing 37.5% C, 12.5% H, and 50.0% O by weight. • Deduce the molecular formula of the compound if the molecular mass is 64. • Empirical formula mass = 12 + 4 + 16 = 32 • n = molecular mass/empirical formula mass 64/32 = 2 Molecular formula is (CH4O) X 2 C2H8O2 END OF PART 2