Introduction to Physiology: The Cell and General Physiology
advertisement

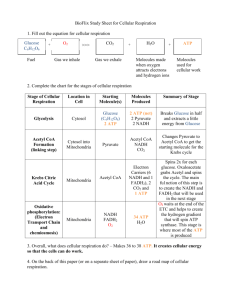
Adenosine triphosphate (ATP) - the central link between energy-producing and energy-using systems of the body Figure 67-1; Guyton & Hall Copyright © 2006 by Elsevier, Inc. ATP Structure Downloaded from: StudentConsult (on 1 March 2009 09:39 PM) Copyright © 2006 by Elsevier, Inc. © 2005 Elsevier Phosphate Terminology • Kinase – adds a phosphate • phosphatase – removes a phosphate • phosphorylase – splits a compound by adding a phosphate (analagous to hydrolysis, but uses phosphate instead of water) Copyright © 2006 by Elsevier, Inc. Glucose Transport Into Most Cells • Down a concentration gradient by facilitated diffusion, – i.e. a carrier is required but energy is not • There are many different carriers. The most important and commonly studied are – GLUT-1, does not require insulin • note that most post-absorptive glucose uptake by cells does not require insulin – GLUT-4, insulin dependent Copyright © 2006 by Elsevier, Inc. Glycolysis 2 ATP Glucose Dihydroxyacetone Phosphate (DHAP) Fructose 1,6diphosphate (PP) 4 ADP Glyceraldehyde 3-phosphate (GA 3-P) 2 NAD+ 2 Pyruvic Acid Net Output: 2 pyruvate 2 NADH + H (4 H’s) 2 ATP Copyright © 2006 by Elsevier, Inc. 4 ATP 2 NADH + 2H Go from 3-C pyruvate to ... O O H3 C - C - C Pyruvic Acid Formation of Acetyl CoA from Pyruvic Acid OH Transport into Mitochrondrion HS - CoA CO2 CO2 NADH + H ... a 2-C Acetyl CoA O H3C - C - S - CoA Acetyl CoA Copyright © 2006 by Elsevier, Inc. Remember, there are 2 pyruvates per glucose molecule! Acetyl CoA Remember... 2 of these per molecule of glucose, so double the outputs of the TCA shown here. O H3C - C - S - CoA Citrate (6-C) O O O C-C-C-C NADH + H HO NADH + H OH Oxaloacetate (4-carbon) NADH + H ATP FADH2 CO2 CO2 Copyright © 2006 by Elsevier, Inc. One molecule of glucose yields... • Glycolysis – 2 NADH – 2 ATP • Pyruvate to Acetyl CoA conversion – 2 NADH (because there are 2 pyruvates) • Citric Acid cycle (numbers are for 2 pyruvates going through) – 6 NADH – 2 FADH2 – 2 ATP • Total: 10 NADH, 2 FADH2, and 4 ATP Copyright © 2006 by Elsevier, Inc. Electron Transport, Making ATP • Each NADH + H yields 2 electrons – Electron transport along the cytochrome chain enables establishment of an electrochemical H+ gradient along the inner mitochondrial membrane. • Hydrogen movement down this gradient, through the ATP synthetase, provides the energy for conversion of ADP to ATP. • Each electron pair from each NADH + H can provide enough energy for production of 3 ATP – electron pair from FADH2 yield 2 ATP Copyright © 2006 by Elsevier, Inc. Chemiosmosis Downloaded from: StudentConsult (on 1 March 2009 09:39 PM) Copyright © 2006 by Elsevier, Inc. © 2005 Elsevier ATP Synthase Copyright © 2006 by Elsevier, Inc. One molecule of glucose yields... • Glycolysis – 2 NADH + 2H 6 ATP – 2 ATP • Pyruvate to Acetyl CoA conversion – 2 NADH + 2H 6 ATP • Citric Acid cycle – 6 NADH + 6H 18 ATP – 2 FADH2 4 ATP – 2 ATP • Total: 38 ATP, which yields ~ 456 kcal Copyright © 2006 by Elsevier, Inc. galactokinase Galactose Galactose-1-P Glucose-1-P glucokinase (liver) Glucose Glucose-6-P hexokinase Fructose-6-P Fructose-1,6-PP Fructose DHA-P Fructose-1-P fructokinase Copyright © 2006 by Elsevier, Inc. GA 3-P Because the liver has this enzyme, it can convert the other monosaccharides into glucose for export. (Renal tubular and intestinal epithelial cells also have this enzyme) Galactose Glucose-1-P glucose-6-phosphatase Glucose Glucose-6-P Fructose-6-P Fructose Phosphorylation of the monosaccharides upon entering cells of the body “traps” it there for use. The G-6-P enzyme is needed for tissues that send glucose to other parts of the body. Copyright © 2006 by Elsevier, Inc. Glycogenesis & Glycogenolyis Glycogen UDP -Glucose Glycogenolysis Glycogenesis Glucose 1-P From the Blood From Gluconeogenesis Copyright © 2006 by Elsevier, Inc. Glucose Glucose 6-P Glycolysis The Lactate Story • The NADH formed from oxidizing glucose eventually gets oxidized back to NAD+ in the mitochondria. – (the result of giving the electrons to electron transport chain) • NAD+ is needed to keep oxidizing glucose. • In exercise (once the anaerobic threshold is crossed), NAD+ isn’t re-formed fast enough, so low levels threaten to stop glycolysis and ATP production. • The main reason to form lactate is to regenerate the NAD+ needed to continue oxidizing glucose. Copyright © 2006 by Elsevier, Inc. GLYCOLYSIS Glyceraldehyde - 3 - P Lactate NAD+ (oxidized) oxidation reaction reduction reaction NADH (reduced) 1,3 - Diphosphoglycerate Copyright © 2006 by Elsevier, Inc. Pyruvate The Fate of Lactate • Lactate is transported to the liver for conversion back to pyruvate and then, via gluconeogenesis, to glucose. – Why would muscle transport lactate to the liver for conversion back to pyruvate? NAD+ is needed for that step, and the point of making lactate in the first place was because NAD+ was too low. • Lactate is in a sense a “storage form” of NADH, because when it gets oxidized back to pyruvate, NADH is formed. • The glucose is released and taken up by the active muscle. Copyright © 2006 by Elsevier, Inc. More on Lactate • Reconversion of Lactic Acid: Why does excess ATP cause excess pyruvate to be converted back to glucose? – This is because high ATP levels inhibit glycolysis and actually promote the reverse, i.e. gluconeogenesis. • Lactic Acid and the Heart – The very high blood flow and O2 levels mean no shortage of NAD+ so any lactate that is formed can be oxidized readily to pyruvate. – Significant lactic acid is formed in the heart only during ischemia. – Lactate Dehydrogenase (LD) has a very low affinity for pyruvate in the heart, also explaining why little lactate is formed normally. However, cardiac LD has high affinity for lactate, hence the ability of the heart to utilize lactate from the circulation during exercise. • in “white” skeletal muscle, LD has a high affinity for pyruvate. Copyright © 2006 by Elsevier, Inc. Control of Glucose Oxidation fructose 1,6-diphosphate fructose 6-P Phosphofructokinase + glucagon ATP citrate Mitochondrion Pyruvate kinase NADH citrate formation (+ other steps) Pyruvate pyruvate dehydrogenase Acetyl CoA Copyright © 2006 by Elsevier, Inc. PEP ADP Pyruvate Effects of Epinephrine and Glucagon Glycogen Phosphorylase a active P Glucose-1-P Activates this by adding P Epinephrine/ Glucagon “Glucose” Copyright © 2006 by Elsevier, Inc. + cAMP-dependent protein kinase Inactivates this by adding P Glycogen Synthase INACTIVE (D) P Glycogen Epinephrine and Glucagon stimulate glycogen breakdown ultimately by adding a P to and activating glycogen phosphorylase (phosphorylase a). Epinephrine/ Glucagon cAMP cAMP-dependent protein kinase Phosphorylates and activates this enzyme P active phosphorylase b kinase Phosphorylase b inactive P Glycogen Phosphorylase a P active Phosphorylase Phosphatase Glucose-1- P Copyright © 2006 by Elsevier, Inc. Final Enzymes for Glycogen -esis and -olysis Glycogenolysis Glycogen Phosphorylase a active P (Phosphorylase b is the inactive form) Glucose-1-P Note that the enzyme for glycogenolysis is activated when it is phosphorylated (i.e. has a phosphate added P ), but the enzyme for glycogenesis is inhibited by phosphorylation. “Glucose” Glycogen Synthase active (I) Glycogen Glycogenesis Copyright © 2006 by Elsevier, Inc.