Chapter 27-28 - Bakersfield College
advertisement

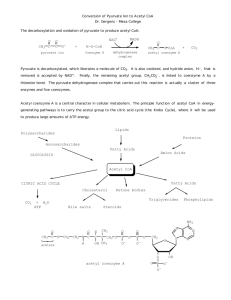
Chemistry B11 Chapter 27 & 28 Metabolic pathway & Energy production Metabolism Chemical reactions in cells that break down or build molecules. It produces energy and provide substances to cell growth. Catabolic reactions: Complex molecules Simple molecules + Energy Anabolic reactions: Simple molecules + Energy (in cell) Complex molecules Metabolism in cell Mitochondria Proteins Urea NH4+ Amino acids e Carbohydrates Polysaccharides Glucose Fructose Galactose Glucose Pyruvate Acetyl CoA Citric Acid cycle e CO2 & H2O Glycerol Lipids Fatty acids Stage 1: Digestion and hydrolysis Stage 2: Degradation and some oxidation (Formation of Acetyl CoA) Stage 3: Oxidation to CO2, H2O and energy Cell Structure Nucleus Membrane Mitochondria Cytoplasm (Cytosol) Cell Structure Nucleus: consists the genes that control DNA replication and protein synthesis of the cell. Cytoplasm: consists all the materials between nucleus and cell membrane. Cytosol: fluid part of the cytoplasm (electrolytes and enzymes). Mitochondria: energy producing factories. Enzymes in matrix catalyze the oxidation of carbohydrates, fats , and amino acids. Produce CO2, H2O, and energy. ATP and Energy - Adenosine triphosphate (ATP) is produced from the oxidation of food. - Has a high energy. - Can be hydrolyzed and produce energy. ph os phoric ester O O O O-P-O-P-O-P-O-CH2 O O O- O H H H ph os phoric 3 Phosphates anh yd rides HO OH NH2 N N N aden ine N -N -glycos idic b on d H -D-ribofuranose Ribose ATP and Energy O O O-P-O-P-O-AMP + H2 O O O- ATP O O-P-O-AMP + H2 PO4 - + 7.3 kcal/mol O AD P (adenosine triphosphate) Pi (adenosine diphosphate) (inorganic phosphate) - We use this energy for muscle contraction, synthesis an enzyme, send nerve signal, and transport of substances across the cell membrane. - 1-2 million ATP molecules may be hydrolysis in one second (1 gram in our cells). - When we eat food, catabolic reactions provide energy to recreate ATP. ADP + Pi + 7.3 kcal/mol ATP Stage 1: Digestion Convert large molecules to smaller ones that can be absorbed by the body. Carbohydrates Lipids (fat) Proteins Digestion: Carbohydrates Salivary amylase Mouth Dextrins + Polysaccharides + Maltose Stomach Small intestine pH = 8 pH = 2 (acidic) Dextrins α-amylase (pancreas) Maltose Lactose Sucrose Bloodstream Glucose Maltase Lactase Sucrase Glucose Glucose + Galactose Glucose + Fructose Liver (convert all to glucose) Glucose + Digestion: Lipids (fat) Small intestine H2C Fatty acid HC Fatty acid H2C Fatty acid + 2H2O Triacylglycerol H2C lipase (pancreas) OH H2C OH HC Fatty acid + 2 Fatty acids Monoacylglycerol Intestinal wall Monoacylglycerols + 2 Fatty acids → Triacylglycerols Protein Lipoproteins Chylomicrons Lymphatic system Bloodstream Cells Enzymes hydrolyzes Glycerol + 3 Fatty acids liver Glucose Digestion: Proteins Pepsinogen HCl Pepsin Stomach Proteins denaturation + hydrolysis Polypeptides Small intestine Trypsin Chymotrypsin Polypeptides Intestinal wall Bloodstream Cells hydrolysis Amino acids Some important coenzymes oxidation Coenzyme + Substrate Coenzyme(+2H) + Substrate(-2H) Reduced 2 H atoms 2H+ + 2e- NAD+ Coenzymes Oxidized FAD Coenzyme A NAD+ Nicotinamide adenine dinucleotide + The p lus sign on N A D represents th e positive ch arge on this n itrogen O CNH2 O - O-P-O-CH2 O ADP AMP H H H HO (Vitamin B3) N+ O H N icotinamide; derived from niacin (vitamin) OH Ribose a -N-glycosidic bond NAD+ - Is an oxidizing agent. - Participates in reactions that produce (C=O) such as oxidation of alcohols to aldehydes and ketones. O CH3-CH2-OH + NAD+ CH3-C-H + NADH + H+ NAD+ + 2H+ + 2e- NADH + H+ H O C NH2 + H+ + 2 e- NH2 : + H H O C N Ad NAD + N Ad N AD H FAD Flavin adenine dinucleotide O H3 C N H3 C N N N Riboflavin CH2 (Vitamin B2) H C OH H C OH H C OH CH2 O O=P-O-AMP - O ADP H Flavin O Ribitol (sugar alcohol) FAD - Is an oxidizing agent. - Participates in reaction that produce (C=C) such as dehydrogenation of alkanes. H H R-C-C-R + FAD HH R-C=C-H + FADH2 H H O H3 C H3 C N N N Ad FAD NH O H3 C H N O + 2 H+ + 2 e H3 C N N Ad H FAD H2 NH O Coenzyme A (CoA) Coenzyme A Aminoethanethiol ( vitamin B5) Coenzyme A (CoA) O O - It activates acyl groups (RC-), particularly the Acetyl group (CH3C-). O O CH3-C- + HS-CoA CH3-C-S-CoA Acetyl group Coenzyme A Acetyl CoA Metabolism in cell Mitochondria Proteins Urea NH4+ Amino acids e Carbohydrates Polysaccharides Glucose Fructose Galactose Glucose Pyruvate Acetyl CoA Citric Acid cycle e CO2 & H2O Glycerol Lipids Fatty acids Stage 1: Digestion and hydrolysis Stage 2: Degradation and some oxidation (Formation of Acetyl CoA) Stage 3: Oxidation to CO2, H2O and energy Stage 2: Formation of Acetyl CoA Glycolysis: Oxidation of glucose - We obtain most of our energy from glucose. - Glucose is produced when we digest the carbohydrates in our food. - We do not need oxygen in glycolysis (anaerobic process). 2 ADP + 2Pi C6H12O6 + 2 NAD+ 2 ATP O 2CH3-C-COO- + 2 NADH + 4H+ Glucose Pyruvate Inside of cell (Cytoplasm) Pathways for pyruvate - Pyruvate can produce more energy. Aerobic conditions: if we have enough oxygen. Anaerobic conditions: if we do not have enough oxygen. Aerobic conditions - Pyruvate is oxidized and a C atom remove (CO2). - Acetyl is attached to coenzyme A (CoA). - Coenzyme NAD+ is required for oxidation. OO O CH3-C-C-O- + HS-CoA + NAD+ pyruvate Coenzyme A CH3-C-S-CoA + CO2 + NADH Acetyl CoA Important intermediate product in metabolism. Anaerobic conditions - When we exercise, the O2 stored in our muscle cells is used. - Pyruvate is reduced to lactate. - Accumulation of lactate causes the muscles to tire and sore. - Then we breathe rapidly to repay the O2. - Most lactate is transported to liver to convert back into pyruvate. OO CH3-C-C-O- NADH + H+ NAD+ HO O CH3-C-C-OH pyruvate Lactate Reduced Glycogen - If we get excess glucose (from our diet), glucose convert to glycogen. - It is stored in muscle and liver. - We can use it later to convert into glucose and then energy. - When glycogen stores are full, glucose is converted to triacylglycerols and stored as body fat. Metabolism in cell Mitochondria Proteins Urea NH4+ Amino acids e Carbohydrates Polysaccharides Glucose Fructose Galactose Glucose Pyruvate Acetyl CoA Citric Acid cycle e CO2 & H2O Glycerol Lipids Fatty acids Stage 1: Digestion and hydrolysis Stage 2: Degradation and some oxidation (Formation of Acetyl CoA) Stage 3: Oxidation to CO2, H2O and energy Step 3: Citric Acid Cycle - Is a central pathway in metabolism. - Uses acetyl CoA from the degradation of carbohydrates, lipids, and proteins. - Two CO2 are given off. - There are four oxidation steps in the cycle provide H+ and electrons to reduce FAD and NAD+ (FADH2 and NADH). 8 reactions Reaction 1 Formation of Citrate O Acetyl CoA CH3-C-S-CoA + COOC=O Oxaloacetate CH2 COO- COOCH2 H2O HO C COO- + CoA-SH CH2 COOCitrate Coenzyme A Reaction 2 Isomerisation to Isocitrate - Because the tertiary –OH cannot be oxidized. (convert to secondary –OH) HO COO- COO- CH2 CH2 C COO- CH2 COOCitrate Isomerisation H C COO- HO C H COOIsocitrate Reaction 3 First oxidative decarboxylation (CO2) - Oxidation (-OH converts to C=O). - NAD+ is reduced to NADH. - A carboxylate group (-COO-) is removed (CO2). H HO COO- COO- COO- CH2 CH2 CH2 C CH2 -COO- - NAD + NADH + H+ COO- H COO- C H C-COO isocitrate H COO - dehydrogenase O CHO CHC Isocitrate -COOCOO - CO CH -COO CH2 -COO 2 2 Isocitrate H C-H H C-COO - - CH2 CO2 O C COO- α-Ketoglutrate Reaction 4 Second oxidative decarboxylation (CO2) - Coenzyme A convert to succinyl CoA. - NAD+ is reduced to NADH. - A second carboxylate group (-COO-) is removed (CO2). COOCH2 2 CHCH -COO 2 O CHC 2 COO-O C-COO α-Ketoglutrate -Ketoglutarate COOCH2 CoA -SH N AD + N AD H -ketoglutarate dehydrogenase complex CH2 CH2 -COO + CO O C CH + CO 2 2 2 S-CoA O C SCoA Succinyl CoA Succinyl-CoA Reaction 5 Hydrolysis of Succinyl CoA - Energy from hydrolysis of succinyl CoA is used to add a phosphate group (Pi) to GDP (guanosine diphosphate). - The hydrolysis of GTP is used to add a Pi to ADP to produce ATP. GTP + ADP → GDP+ ATP COOCH2 CH2 + H2O + GDP + Pi O C COOCH2 CH2 COO- S-CoA Succinyl CoA Succinate + GTP + CoA-SH Reaction 6 Dehydrogenation of Succinate - H is removed from two carbon atoms. - Double bond is produced. - FAD is reduced to FADH2. COOCH2 CH2 -COO CHCH 2 -COO 2 COO Succinate Succinate COOFAD H FAD H2 succinate dehydrogenas e - C CH COO C CH OOC H COOFumarate Fumarate Reaction 7 Hydration - Water adds to double bond of fumarate to produce malate. COOCH CH COOFumarate H 2O COOHO C H CH2 COOMalate Reaction 8 Dehydrogenation forms oxaloacetate - -OH group in malate is oxidized to oxaloacetate. - Coenzyme NAD+ is reduced to NADH + H+. COOHO CHCOO H HO C CH2 -COO CH2 L-Malate COOMalate COON AD + N AD H + HO+ C-COO C=O CH2CH -COO malate 2 dehydrogenase OxaloacetateCOO Oxaloacetate Summary The catabolism of proteins, carbohydrates, and fatty acids all feed into the citric acid cycle at one or more points: Citric Acid Cycle Summary Summary Summary The main function of the citric acid cycle is to produce reduced coenzymes (NADH and FADH2). These molecules enter the electron transport chain (Stage 4) and ultimately produce ATP. Feedback Mechanism The rate of the citric acid cycle depends on the body’s need for energy. When energy demands are high and ATP is low → the cycle is activated. When energy demands are low and NADH is high → the cycle is inhibited. Stage 4: Electron Transport & Oxidative Phosphorylation - Most of energy generated during this stage. - It is an aerobic respiration (O2 is required). 1. Electron Transport Chain (Respiratory Chain) 2. Oxidative Phosphorylation Electron Transport H+ and electrons from NADH and FADH2 are carried by an electron carrier until they combine with oxygen to form H2O. FMN (Flavin Mononucleotide) Fe-S clusters Electron carriers Coenzyme Q (CoQ) Cytochrome (cyt) FMN (Flavin Mononucleotide) H O Riboflavin (Vitamin B2) H3 C N H3 C N N N O H Flavin O 2H+ + 2e- CH2 H3 C N H3 C N Riboflavin H C OH H C OH H C OH N N H C OH CH2 CH2 O O=P-O-AMP O O=P-O-AMP - - O O FMN + 2H+ + 2e- → FMNH2 Reduced Flavin O CH2 H H C OH H C OH Ribitol (sugar alcohol) H Ribitol Fe-S Clusters Cys S S S Cys S + 1 e- Fe3+ Cys Cys S Cys Fe3+ + 1e- S Cys S Cys Fe2+ Cys S Fe2+ Reduced Coenzyme Q (CoQ) OH 2H+ + 2e- OH Coenzyme Q Reduced Coenzyme Q (QH2) Q + 2H+ + 2e- → QH2 Reduced Cytochromes (cyt) - They contain an iron ion (Fe3+) in a heme group. - They accept an electron and reduce to (Fe2+). - They pass the electron to the next cytochrome and they are oxidized back to Fe3+. Fe3+ + 1eOxidized Fe2+ Reduced cyt b, cyt c1, cyt c, cyt a, cyt a3 Electron Transfer Mitochondria Electron Transfer Complex I NADH + H+ + FMN → NAD+ + FMNH2 FMNH2 + Q → QH2 + FMN NADH + H+ + Q → QH2 + NAD+ Complex II FADH2 + Q → FAD + QH2 Electron Transfer Complex III QH2 + 2 cyt b (Fe3+) → Q + 2 cyt b (Fe2+) + 2H+ Complex IV Aerobic 4H+ + 4e- + O2 → 2H2O From reduced coenzymes or the matrix From the electron transport chain From inhaled air Oxidative Phosphorylation Transport of electrons produce energy to convert ADP to ATP. ADP + Pi + energy → ATP + H2O Chemiosmotic model - H+ make inner mitochondria acidic. - Produces different proton gradient. - H+ pass through ATP synthase (a protein complex). ATP synthase Total ATP Glycolysis: 7 ATP Oxidation of Pyruvate: 5 ATP Citric acid cycle: 20 ATP Oxidation of glucose 32 ATP C6H12O6 + 6O2 + 32 ADP + 32 Pi → 6CO2 + 6H2O + 32 ATP Metabolism in cell Mitochondria Proteins Urea NH4+ Amino acids e Carbohydrates Polysaccharides Glucose Fructose Galactose Glucose Pyruvate Acetyl CoA Citric Acid cycle e CO2 & H2O Glycerol Lipids Fatty acids Step 1: Digestion and hydrolysis Step 2: Degradation and some oxidation Step 3: Oxidation to CO2, H2O and energy Oxidation of fatty acids α O CH3-(CH2)14-CH2-CH2-C-OH oxidation - Oxidation happens in step 2 and 3. - Each beta oxidation produces acetyl CoA and a shorter fatty acid. - Oxidation continues until fatty acid is completely break down to acytel CoA. Oxidation of fatty acids Fatty acid activation - Before oxidation, they activate in cytosol. O O R-CH2-C-OH + ATP + HS-CoA Fatty acid R-CH2-C-S-CoA + H2O + AMP + 2Pi Fatty acyl CoA -Oxidation: 4 reactions Reaction 1: Oxidation (dehydrogenation) HHO O R-CH2-C-C-C-S-CoA + FAD H H R-CH2-C=C-C-S-CoA + FADH2 H H Fatty acyl CoA Reaction 2: Hydration O R-CH2-C=C-C-S-CoA + H2O H H HO H O R-CH2-C-C-C-S-CoA H H Reaction 3: Oxidation (dehydrogenation) HO H O O R-CH2-C-C-C-S-CoA + NAD+ O R-CH2-C-CH2-C-S-CoA + NADH+ H+ H H Reaction 4: Cleavage of Acetyl CoA O O R-CH2-C-CH2-C-S-CoA + CoA-SH O O R-CH2-C-S-CoA + CH3-C-S-CoA Fatty acyl CoA Acetyl CoA Oxidation of fatty acids One cycle of -oxidation O R-CH2-CH2-C-S-CoA + NAD+ + FAD + H2O + CoA-SH O O R-C-S-CoA + CH3-C-S-CoA + NADH + H+ + FADH2 Fatty acyl CoA # of Acetyl CoA = Acetyl CoA # of fatty acid carbon 2 = 1 + oxidation cycles Ketone bodies - If carbohydrates are not available to produce energy. - Body breaks down body fat to fatty acids and then Acetyl CoA. - Acetyl CoA combine together to produce ketone bodies. - They are produced in liver. - They are transported to cells (heart, brain, or muscle). O CH3-C-S-CoA O CH3-C-S-CoA Acetyl CoA Acetone O O O CH3-C-CH2-C-O- CH3-C-CH3 + CO2 + energy OH Acetoacetate O CH3-CH-CH2-C-O-Hydroxybutyrate Ketosis (disease) - When ketone bodies accumulate and they cannot be metabolized. - Found in diabetes and in high diet in fat and low in carbohydrates. - They can lower the blood pH (acidosis). - Blood cannot carry oxygen and cause breathing difficulties. Fatty acid synthesis - When glycogen store is full (no more energy need). - Excess acetyl CoA convert to 16-C fatty acid (palmitic acid) in cytosol. - New fatty acids are attached to glycerol to make triacylglycerols. (are stored as body fat) Metabolism in cell Mitochondria Proteins Urea NH4+ Amino acids e Carbohydrates Polysaccharides Glucose Fructose Galactose Glucose Pyruvate Acetyl CoA Citric Acid cycle e CO2 & H2O Glycerol Lipids Fatty acids Stage 1: Digestion and hydrolysis Stage 2: Degradation and some oxidation (Formation of Acetyl CoA) Stage 3: Oxidation to CO2, H2O and energy Degradation of amino acids - They are degraded in liver. Transamination: - They react with α-keto acids and produce a new amino acid and a new α-keto acid. + NH3 CH3-CH-COO- O + + NH3 O pyruvate 2-CH2-COO α-ketoglutarate alanine CH3-C-COO- -OOC-C-CH + -OOC-CH-CH -CH -COO2 2 glutamate Degradation of amino acids Oxidative Deamination + NH3 -OOC-CH-CH -CH -COO2 2 + H2O + NAD+ glutamate dehydrogenase glutamate O -OOC-C-CH 2-CH2-COO α-ketoglutarate + NH4+ + NADH + H+ Urea cycle - Ammonium ion (NH4+) is highly toxic. - Combines with CO2 to produce urea (excreted in urine). - If urea is not properly excreted, BUN (Blood Urea Nitrogen) level in blood becomes high and it build up a toxic level (renal disease). - Protein intake must be reduced and hemodialysis may be needed. O 2NH4+ + CO2 H2N-C-NH2 + 2H+ + H2O urea Energy from amino acids - C from transamination are used as intermediates of the citric acid cycle. - amino acid with 3C: pyruvate - amino acid with 4C: oxaloacetate - amino acid with 5C: α-ketoglutarate - 10% of our energy comes from amino acids. - But, if carbohydrates and fat stores are finished, we take energy from them.