name
advertisement

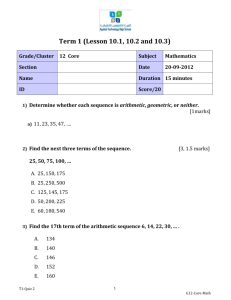
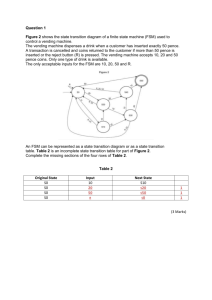
NAME: ……………………………………………… INDEX NO ……..…................................... CANDIDATE’S SIGN ……………………………….….... DATE: ………………………..………… 233/2 CHEMISTRY PAPER 2 JULY/AUGUSTY-2015 TIME: 2 HOURS Kenya Certificate of Secondary Education (K.C.S.E.) 233/2 CHEMISTRY PAPER 2 JULY/AUGUSTY-2015 TIME: 2 HOURS INSTRUCTIONS TO CANDIDATES: Write your name and index number in the spaces provided above Sign and write the date of examination in the spaces provided above. Answer all the questions in the spaces provided Mathematical table and silent calculators may be used All the working must be clearly shown where necessary This paper consists of 7 questions. FOR EXAMINER’S USE ONLY: SECTION QUESTIONS MAXIMUM SCORE A 1 2 3 4 5 6 7 TOTAL 14 11 11 11 10 13 B CANDIDATE’S SCORE 80 This paper consists of 8 printed pages. Candidates should check the question paper to ascertain all the pages are printed as indicated And no questions are missing. 1. Excess zinc granules was added to a beaker containing 100cm3 of dilute sulphuric(vi) acid. The beaker © 2015 Khwisero Sub-county Form Four 1 233/2 Chemistry Turn Over was then placed on a balance and the total loss in mass recorded after every two minutes as shown in the table below. Time (minutes) 0 2 4 6 8 10 12 Total loss in mass (g) 0 7.0 9.6 11.5 13.0 13.7 14.0 14.0 (a) Why was there loss in mass? 14 15 14.0 (1mark) ……………………………………………………………………………………………………....…. (b) Calculate the average rate of loss in mass between (i) 0 and 2 minutes (1mark) (ii) 4 and 6 minutes (1mark) (c) Explain the difference in the average rates in b(i)and b(ii) above (2marks) …………………………………………………………………………………………………..……… ……………………………………………………………………………………………………....…. (d)Write a balanced equation for the reaction rate above could be increase. (3marks) …………………………………………………………………………………………………..……… (f) (i) Plot the graph of total loss in mass(g) versus time (minutes). (ii) Use the graph to calculate the rate of the reactions when t = 5 minutes. (3marks) (2marks) 2. (a) State how burning can be used to distinguish between prepane and propyne. Explain your answer. (3marks) © 2015 Khwisero Sub-county Form Four 2 233/2 Chemistry Turn Over …………………………………………………………………………………………………..……… ……………………………………………………………………………………………………....…. …………………………………………………………………………………………………..……… (b)Draw the structural formular of the 4th member of the homologous series in which propyne is a member (1mark) (c) The flow chart below shows a series of reactions starting with propan-l-ol. Study it and answer the questions that follow. Propanoic acid NaOH C Process Y Sodalime Heat Propan –l-ol Ethane Concentrated H2SO4 Process W Gas R High Pressure Polymer X (i) 3. Name: I. Process Y……………………………………………………….. (1mark) II. Substances R and C R……………………………………………….. (1mark) C……………………………………………….. (1mark) III. Process W……………………………………………… (1mark) IV. Polymer X……………………………………………… (1mark) (ii) Write a balanced chemical equation for the combustion of propane. (1mark) …………………………………………………………………………………………………..……… (iii) State one use of polymer X (1mark) …………………………………………………………………………………………………..……… The set up below was used to prepare dry hydrogen chloride gas. Liquid Q Sodium chloride (a) Complete the diagram to show how dry hydrogen chloride gas is collected. (b) Identify liquid Q © 2015 Khwisero Sub-county Form Four 3 233/2 Chemistry (2marks) ( 1mark) Turn Over …………………………………………………………………………………………… (c) Write a balanced equation for the reaction that produces hydrogen chloride gas in the above experiment (1mark) …………………………………………………………………………………………………..……… (d) State the effect of dry hydrogen chloride gas on (i) Dry litmus paper ( 1mark) …………………………………………………………………………………………………..……… (ii) Wet litmus paper (1mark) …………………………………………………………………………………………………..……… (e) Calculate the volume of hydrogen chloride gas produced if 120g of sodium chloride was used with excess of liquid Q at S.T.P .( Na= 23,Cl=35.5,H=1.0, S = 3) molar gas volume = 22.4 litres at stp). (3marks) (f) State and explain the observations made when hydrogen chloride gas is bubbled through silver nitrate solution. 4. (2marks) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (a) Use the thermochemical equations below to answer the questions that follow. C2H6(g) + 7/2 O2 (g) 2 CO2(g) + 3H2O(l) H1 = -1560kJmole-1 C(s) + O2(g) CO2(g) H2 = - 394kJmole-1 H2(g) + ½ O2(g) H2O2(l) H3 = - 286kJmole-1 (i) Name the two types of heat changes represented by H3. ( 2 marks) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (ii) Use the data above to calculate the standard enthalpy of formation of ethane. (3marks) (iii) Draw an energy level for the reaction represented by Hf where Hf is the molar heat of formation of ethane. (2marks) (b)When a sample of ethane was burnt , the heat energy evolved raised the temperature of 400g water by 19.5 k ( specific heat capacity of water = 4.2 Jg-1k-1) © 2015 Khwisero Sub-county Form Four 4 233/2 Chemistry Turn Over Calculate the (i) Heat change for the reaction. (2marks) (ii) Mass of ethane that was burnt (Relative formular mass of ethane =30, Hc of ethane = - 1560kJmole-1) ( 2marks) 5. (a) At. 30oC, 54g of potassium nitrate were added to 100g of water to make a super saturated solution. Define a saturated solution. (1mark) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (b) The table below gives the solubilities of potassium nitrate salt at different temperatures. Temperature (oC) 10 20 30 39 45 55 60 Solubility g/100g of water 19 31 44 60 72 98 120 (i) Plot the graph of the solubility of potassium nitrate (vertical) against temperature. (ii) Use your graph to I. Determine the solubility of potassium nitrate at 25oC © 2015 Khwisero Sub-county Form Four 5 (1mark) (3marks) (1mark) 233/2 Chemistry Turn Over II. Determine the mass of potassium nitrate that remained undissolved when 80g of potassium nitrate were added to 100cm3 of water and warmed to 40oC. (2marks) (c) Determine the molar concentration of potassium at 25oC ( K = 39, N= 14,O=16, density of water = 1.0g(cm3) 6. The grid below is a section of the periodic table , the letters are not the actual chemical symbols. Use it to answer the questions that follow. X W Z T R Y D A (a) Write a balanced chemical equation for the reaction between T and Steam ( 1mark) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (b) State the nature of the most table oxide of W ( 1mark) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (c) Identify the letter representing an element that (i) Is the most electronegative. ( 1mark) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (ii) Loses electrone most readily ( 1mark) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (iii) Forms an oxide that reacts with both aqueous solutions of sodium hydroxide and hydrochloric acid. (1mark) …………………………………………………………………………………………………..……… © 2015 Khwisero Sub-county Form Four 6 233/2 Chemistry Turn Over (d) Write a balanced equation to show the effect of heat on the (i) Nitrate of A (1mark) …………………………………………………………………………………………………..……… (ii) Carbonate of T (1mark) …………………………………………………………………………………………………..……… (e) Distinguish between a strong and weak acids, give one example of each one of them. (3marks) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… ( f) (i) Define Neutralization (1mark) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (ii) Starting with Copper (II) oxide, describe the laboratory preparation of copper (ii) sulphate crystals (3marks) …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… ………………………………………………………………………………………………….…....… 7. Sodium carbonate is an important industrial chemical which is obtained industrially as illustrated in the flow chart below. Use it to answer the questions that follow. (a) (i) Name the substance W (1mark) ………………………………………………………………………………………………….…....… (ii) Coldwater is made to circulate around B. What does this suggest about the reaction of ammonia gas with brine. (1mark) (iii) Name three main starting materials required in the manufacture of sodium carbonate (1½ marks) ………………………………………………………………………………………………….…....… © 2015 Khwisero Sub-county Form Four 7 233/2 Chemistry Turn Over …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (iv) Explain how sodium carbonate crystals may be obtained from sodium hydrogen carbonate. (1mark) ………………………………………………………………………………………………….…....… …………………………………………………………………………………………………..……… b) (i) Identify three by products that can be recycled in this industrial process (1 ½ marks) ………………………………………………………………………………………………….…....… …………………………………………………………………………………………………..……… ………………………………………………………………………………………………….…....… (ii) Name the by- product produced in this process and state its use. (2marks) ………………………………………………………………………………………………….…....… …………………………………………………………………………………………………..……… (iii) Name the process taking place at G that enable the two compounds to separate on cooling. ( 1mark) ………………………………………………………………………………………………….…....… …………………………………………………………………………………………………..……… (iv) Why is it not possible to prepare potassium hydrogen carbonate by a process like the one above? (1mark) ………………………………………………………………………………………………….…....… …………………………………………………………………………………………………..……… © 2015 Khwisero Sub-county Form Four 8 233/2 Chemistry Turn Over