Lecture Packet#4

Humidity, Condensation and Clouds
Chapter 4
Humidity
• The term humidity is used to describe the amount of water vapor in the air.
• Water vapor in the atmosphere is extremely important. Without it there would be no clouds and no precipitation.
• If all the water vapor in the atmosphere were to suddenly condense and fall as rain, it would cover the earth’s surface with about 1 inch of water.
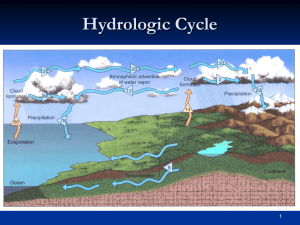
Circulation of water in the
Atmosphere
• Within the atmosphere there is an unending circulation of water.
• Oceans occupy 70% of the earth’s surface; we can speak of the circulation of water as beginning over the ocean.
• Evaporation: transformation of liquid water into water vapor
• Condensation: water vapor changes back into liquid (this forms our clouds)
• Precipitation: liquid (or solid) cloud particles grow in size and fall to the earth’s surface
85% evap from here
Remaining 15% from land
The Hydrological cycle: “Water cycle”
Water molecules travel from the ocean to the atmosphere to the land and back to the ocean
Transpiration: process that allows plants to give up moisture. Water absorbed by roots, moves through stem system and emerges from the plant through the underside of leaves.
Evaporation – A closer look
• Water molecules at the surface of the water are evaporating and condensing. But more are evaporating so net evaporation is occurring
• Recall temperature of water is a measure of the average speed of the molecules.
Molecules near the surface move fast enough to break away and enter the air.
Saturation
• When the dish is covered eventually the total number of molecules escaping the liquid will be balanced by the number of returning molecules.
• This condition is know as saturated air. (No net loss of water vapor)
• Remove the cover and blow across the jar and evaporation will resume –
Why?
Condensation Nuclei
• If we examine the air above the water in the jar of the previous pictures, we would find that the air molecules are mixed with tiny (microscopic) bits of dust, smoke, ocean salt, etc.
• Since these all serve as surfaces on which water vapor may condense they are called condensation nuclei.
• Condensation is more likely to occur when air is cooled because the speed of the water vapor decreases.
Which “holds” more moisture?
Warm or Cold Air
• Although condensation is more likely to occur when the air cools, no matter how cold it becomes, there are always a few molecules with sufficient speed (energy) to remain as a vapor.
• Given the same number of water vapor molecules in the air, saturation is more likely to occur in cool vice warm air.
• Thus, warm air can “hold” more water vapor molecules before becoming saturated than can cold air.
Humidity
• Any one of a number of ways of expressing the amount of water vapor in air.
• Absolute humidity : or water vapor density. Comparison of the mass of water vapor with the volume of air in the parcel
(g/m³)
• Specific Humidity : mass of water vapor in parcel compared to total mass of air in parcel (g/kg)
• Mixing Ratio : mass of water vapor in the parcel compared to the mass of the remaining dry air in the parcel (g/kg)
Vapor Pressure
• Suppose our previous air parcel is near sea level and the air pressure inside the parcel is
1000 mb. (Total air pressure inside parcel is due to collision of all molecules against wall of parcel)
• Parcel is 78% nitrogen, 21% oxygen and 1% water vapor. Pressure of nitrogen is 780 mb, oxygen 210 mb and water vapor is 10 mb.
• So, the partial pressure of the water vapor
(actual vapor pressure) is 10 mb.
Saturation Vapor Pressure
• All else equal, the more air molecules in a parcel the greater the total air pressure. (Think of blowing up a balloon).
• Similarly, increase the amount of water vapor molecules and increase the vapor pressure.
• Where vapor pressure indicates the total amount of water vapor in the air, saturation vapor pressure describes how much water vapor is necessary to make the air saturated at a given temperature
• Example: SVP increases with increasing temperature. At 10 o C the SVP is 12 mb whereas at 30 o C it is 42 mb. SVP over water is greater than over ice.
Relative Humidity
When the air is cool (morning), the relative humidity is high. When the air is warm (afternoon), the relative humidity is low. These conditions exist in clear weather when the air is calm or of constant wind speed.
• Most commonly used way to describe atmospheric moisture. Also the most misunderstood.
• RH=water vapor content/water vapor capacity
• Or RH= actual vapor pressure/saturation vapor pressure (expressed as a percent) x 100%
• Simply put it is the ratio of the air’s water vapor content to its capacity
• Change in RH brought about in two primary ways:
– Changing the air’s water vapor content
– Changing the air temperature
• Note normal times of high vs low RH
.
RH and Dew Point
• Suppose we go out early one morning.
The air temperature is 50 o F (10 o C) and the
RH is 100%.
• We know the svp at 10 o C is 12mb (recall svp slide). Since we know the air is saturated (100% RH) we also must know that the actual vapor pressure must also be 12mb; RH=12mb(vp)/12mb (svp) x
100%
RH and Dew Point
• Suppose as the day progresses the air warms to 30 o C (86 o F) with no change in water vapor content.
• Thus the actual vapor pressure remains
12 mb
• The temperature did increase to 30 o C so the SVP increased to 42 mb
• RH=12 mb (vp)/42 mb (svp) x 100%
• RH is now 29%
RH and Dew Point
• Now a question!
• To what temperature must the outside air in this example (currently 30 o C) be cooled so that it can once again be saturated?
• The answer…..10
o C
• In this example 10 o C is called the dew-point temperature or “dew point”
• Dew point is the temperature to which air would have to be cooled (with no change in air pressure or moisture content) for saturation to occur.
Average surface dew-point temperatures ( °F) for January.
Average surface dew-point temperatures ( °F) for July.
RH and Dew Point
RH and Dew Point
Heat Index
To calculate the apparent temperature, find the intersection of the air temperature and the RH. 100 o F with RH 60% HI=130F
Sling Psychrometer
• Instrument used to obtain the dew point and RH
•
Wet bulb (white sock)- wick-covered thermometer dipped in clean water
•
Spin the sling and water evaporates from the wick and thermometer cools.
•
Wet bulb temperature
– lowest temperature that can be attained by evaporating water into air.
• Dry bulb gives current air temperature.
• Wet bulb depression – difference between the dry bulb and the wet bulb
– Large depression indicates a great deal of water can evaporate into air and the RH is low
– WB=DB indicates 100% RH (saturated air)
Question to ponder?
Does a volume of humid air weigh the same as a similar volume of dry air?
Dew
• On calm clear nights, objects near the earth surface cool rapidly emitting infrared radiation.
• The ground and objects on it often become much colder than surrounding air.
• Eventually the air cools to the dewpoint.
• As surfaces cool below this temperature, water vapor begins to condense on them forming tiny visible specks of water called DEW
Frost
• Visible white frost forms on cold clear mornings when the dew-point temperature is at or below freezing.
• When the air cools to the dew point (frost point) and further cooling occurs water vapor can change directly to ice without becoming a liquid first
(deposition) forming
FROST.
Haze
• When the air’s RH reaches about 75%, some of its water vapor begins to condense onto tiny floating particles of sea salt and other substances – condensation nuclei that are hygroscopic (water seeking) in that they allow water vapor to condense onto them when the
RH is considerably below 100%.
• As water collects onto these nuclei, their size increases and the particles, although still small, are now large enough to scatter visible light in all directions, becoming haze – a layer of particles dispersed through a portion of the atmosphere.
Fog
• As the RH gradually approaches 100%, the haze particles grow larger, condensation begins on the less active nuclei. Droplets grow bigger, and eventually become visible to the naked eye. When visibility lowers to less than 1 km (.62 mi) and the air is wet with millions of tiny floating water droplets, the haze becomes a cloud resting near the ground
– FOG
Types of Fog
• Radiation fog – produced by the earth’s radiational cooling
• Also called ground fog
• Forms on clear nights when a shallow layer of moist air is near the surface and drier air is above it.
• Winds less than 5 kts are important to aid in forming fog
• Advection fog – forms when warm moist air moves over a colder surface. The moist air cools to its saturation point and fog forms.
• Upslope fog – forms as moist air flows up along an elevated plain, hill or mountain.
(Winter/Spring on east side of
Rockies)
• Evaporation Fog – Warm moist air meets cold air. When you see your breath in the winter.
When sun evaporates rain from an asphalt road.
What type of fog?
What type of fog?
Average annual number of days with dense fog throughout the United States
.
Clouds
• Four Major cloud groups and their types:
• High clouds :
Cirrus, Cirrostratus, Cirrocumulus
• Middle clouds :
Altostratus, Altocumulus
• Low clouds :
Stratus, Stratocumulus, Nimbostratus
• Clouds with vertical development:
Cumulus and Cumulonimbus
Although we divide clouds into levels we categorize them based on their appearance
High Clouds
• Approximate heights (mid latitudes)
16,000 to 43,000 ft
• Tropical Regions: 20,000 to 60,000 ft
• Polar Regions: 10,000 to 26,000 ft
• These clouds are composed of ice crystals and are also rather thin.
• Cirrus, Cirrocumulus, Cirrostratus
Cirrus
• Mare’s tails
• Thin wispy
• Usually move west to east
Cirrocumulus
• Less common than cirrus
• Small, rounded white puffs
• May occur individually or in long rows
• Mackerel sky
Cirrostratus
• Often covers the entire sky
• Thin
• Often produce halo as ice crystals in the cloud bend light passing through them
• Use to predict rain/snow within 24 hours
Middle Clouds
• Approximate height (mid latitudes) – 6500 to 23,000 ft
• Tropical regions: 6500 to 26,000 ft
• Polar Regions: 6500 to 13,000 ft
• Clouds composed of water droplets and when air is colder ice crystals.
• Altocumulus, Altostratus
Altocumulus
• Gray, puffy masses sometimes in waves or bands
• Puffs are larger than cirrocumulus (two finger rule)
Altostratus
• Gray or blue-gray cloud that often covers the entire sky. Sun or moon may been dimly visible
• Altostratus clouds form ahead of storms and bring widespread continuous precipitation
Low Clouds
• Approximate height (mid latitudes) – surface to 6500 ft
• Tropical regions: Surface to 6500 ft
• Polar regions: Surface to 6500 ft
• Cumulus, stratus, stratocumulus, nimbostratus
Nimbostratus
• Dark gray “wet” looking cloud.
• Associated with continuous precipitation (light to moderate)
• Never associated with showers
Stratocumulus
• Low lumpy appearance
• Appears in rows or patches
Stratus
• Uniform, grayish cloud
• Often covers the entire sky
• Resembles fog that does not touch the ground
Cumulus
• Puffy clouds
• Cottony clouds that take on shapes
• White to light gray
• Fair weather Cumulus
Cumulus Congestus
• “Towering Cumulus”
• Cumulus clouds that grew up!
• Rain showers
Cumulonimbus (Cb)
• Thunderstorm
• Anvil tops
• Heavy precipitation, hail, lightning, gusty winds
• Big Cb’s breed tornadoes
• Often top out near the tropopause. Strong ones poke through the tropopause.
Mammatus
Contrails – Vapor trails from jets. Condensation from engine exhaust
Nacreous
“Mother of pearl” clouds form in the stratosphere at altitudes
Above 100,000 ft. Best viewed in polar regions in winter.
Super cooled water? Not sure.
Noctilucent Clouds – “luminous night clouds”
Best viewed in polar regions at night. Composed of ice crystals