Fusion and Fission

FUSION AND FISSION
THE SUN
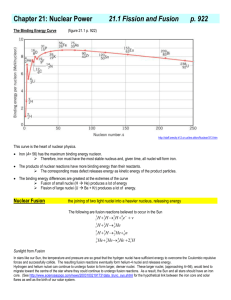
Nuclear Fusion
• Nuclear fusion is the process by which multiple nuclei join together to form a heavier nucleus.
• It is accompanied by the release or absorption of energy depending on the masses of the nuclei involved ..
DEUTERIUM
FUSION
NEUTRON
HELIUM
TRITIUM http://fusioned.gat.com
Nuclear Fusion
• Iron and nickel nuclei have the largest binding energies per nucleon of all nuclei and therefore are the most stable.
Nuclear Fusion
• The fusion of two nuclei lighter than iron or nickel generally releases energy.
• The fusion of nuclei heavier than them absorbs energy.
Complete the Reaction
1
H element atomic number
(protons)
4
Be
1
H
6
C
2
He
1
H
1H
2
He
2
He
6
C
4
Be
8
O
2
He
2
He
Energy needed for Fusion
The thermal activity of a gas is described by its temperature measurement which is really an indication of its velocity/energy.
Thermal energy is represented by the height that the upper magnet.
The upper ring has a potential energy given by PE = mgh at its drop point which is converted into kinetic energy
(KE = 1/2 mv2)
As the magnet falls towards the lower magnet. The two magnets click lightly when the kinetic energy is just greater than the magnetic energy that holds them apart.
Since Kinetic Energy = Potential Energy (ignoring frictional components), the gravitational pull and mass of the upper magnet are constant, then the height needed to overcome the magnetic repelling force is proportional to that magnetic repelling force.
Energy needed for Fusion
PE =mg x h
2
-h
1
PE = KE = Fxh
1
F=force of repulsion
F=PE/h
1
Upper Drop
Position
Ring
Magnets
Float
Position
Lower
Magnet h
1 h
2
Wood Block
Fusion Changes Mass to Energy
E=mc
2
.993 kg Helium
1kg Hydrogen
Cookie Fusion
• Procedure
• Cut 2 squares of wax paper 10 cm on a side
• Cut 5 cm wide slice of cookie dough (atom)
• Find the mass of the atom and record on the table
• Place the atom one cm away from the edge of a wax paper square
• Repeat step 2 thru 4 for a second atom
• Place the atoms about 2 cm from each other
• Place both atoms on a plate and microwave for 1 minute
• Remove the “new element” and let cool for 2 minutes
• Find the mass of the “new element”
• Complete the table
Atom 1
Atom 2
Total
Difference
Cookie Fusion
Mass Before
Cooking
Mass After
Cooking
Learning Check
What process creates energy in the Sun?
Fusion of hydrogen into helium in the Sun’s core generates the Sun’s energy.
How long ago did fusion generate the energy we now receive as sunlight?
Fusion created the energy we receive today about a million years ago. This is the time it takes for photons and then convection to transport energy through the solar interior to the photosphere. Once sunlight emerges from the photosphere, it takes only about 8 minutes to reach Earth.
Learning Check
NUCLEAR FISSION
A reaction in which an atomic nucleus of a radioactive element splits by bombardment from an external source, with simultaneous release of large amounts of energy, used for electric power generation
Nuclear Fission
Neutron induced in U 235
Fission is Exothermic
The sum of the masses of the resulting nuclei is less than the original mass
(about 0.1% less)
The “missing mass” is converted to energy according to E=mc 2
Neutrons may:
1 - Cause another fission by colliding with a U 235 nucleus
• Creates two smaller nuclides and free neutrons
• The free neutrons potentially collide with nearby U 235 nuclei
• May cause the nuclide to split as well
Each split (fission) is accompanied by a large quantity of E-N-E-R-G-Y
2 - Be absorbed in other material
3 - Lost in the system
If sufficient neutrons are present, we may achieve a chain reaction
Source: EIA
U.S. Electrical Power
Production by Source
(2004)
Nuclear Fuel Costs
• Nuclear Fuel Costs Include
– Uranium
– Enrichment
– Manufacturing
– Waste Disposal
• Total Nuclear Fuel Cost is Only About 0.5 cents per kilowatt-hour
– Uranium accounts for only about 20% of this cost or
0.1 cents per kilowatt-hour
– Increasing Uranium Cost has Minimal Impact
Nuclear fusion:
Several small nuclei fuse together and release energy.
Review
Nuclear fission:
A large nucleus splits into several small nuclei when impacted by a neutron, and energy is released in this process
Draw a Double Bubble Map of
Fusion and Fission
fusion fission
Differences Similarities Differences