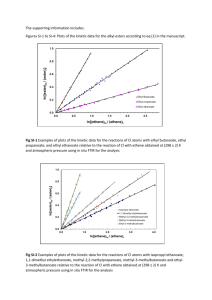
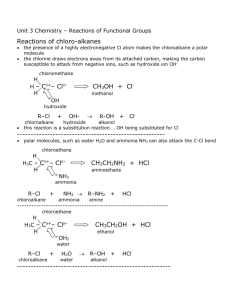
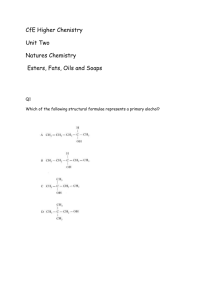
Organic Reactions
advertisement

An alkane molecule such as ethane has a ‘backbone’ consisting of a chain of single C-C bonds. An alkane molecule is non-polar as carbon and hydrogen have similar electronegativity. Therefore they are insoluble in water, but are soluble in non-polar solvents. The stability of the C-C bonds and the nonpolar nature means that alkanes are quite nonreactive. Most reactions involving alkanes are either combustion or substitution reactions. Alkanes can be used as fuel. Combustion reactions involving alkanes release large amounts of heat energy. Combustion of methane › CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + energy Combustion of methane › 2C8H18(g) + 25O2(g) → 16CO2(g) + 18H2O(g) + energy One or more hydrogen atoms in an alkane is replaced by a different atom or functional group. This involves breaking the C-H bonds and making new bonds with the substituting atom or group. Chloroethane is a gas at room temperature and is used in a local anaesthetic spray. Heat or light CH3CH3(g) + Cl2(g) CH3CH2Cl(g) + HCl(g) Page 146 Questions 1 and 2 Alkenes: › Are unsaturated › Are non-polar › Are insoluble in water › Participate in addition reactions › Polymerises to produce polymers. The double covalent bond in ethene molecules has a significant effect on its chemical properties. Ethene reacts more readily, and with more chemicals than ethane. The reactions of ethene usually involve addition of a small molecule to produce a single product. For example it reacts with bromine solution. Involve the C=C bond being converted to a single bond. Ethanol can be produced by an addition reaction of ethene and water using a catalyst to speed up the reaction. A type of addition reaction of ethene is involved in making polyethene. The number n in this reaction is very large (several thousand or more). A molecule made by linking a large number of small molecules, is called a polymer. The small molecule (in this case ethene) is called a monomer. This type of reaction is known as addition polymerisation When the polymer is being formed the ethene molecules add to the end of growing polymer chains. Ethene is used as a base for other addition polymerisation reactions. For example to make PVC and polystyrene. Page 149 Question 1-3 Once a more electronegative atom such as chlorine has been substituted for a hydrogen atom in an alkane, the molecule becomes polar. Electrons in the carbon-chlorine bond are attracted towards the more electronegative chlorine atom. This makes the carbon atom at the other end of the bond susceptible to attack by negatively charge ions. For example chloromethane is converted to methanol when it is reacted with hydroxide ions. The chlorine atom is substituted by an OH functional group to form methanol. Alkanols can be produced by addition reactions of alkenes or substitution reactions of chloroalkanes. Ethanol has very different properties from ethane or chloroethane. › It is liquid at room temp. › It is widely used as a solvent in cosmetics and pharmaceuticals › It is the active ingredient in alcoholic drinks › It can act as a depressant on the human body, slowing reactions and responses. › Excess ethanol consumption also blocks the production of antidiuretic hormones, increasing urination and resulting in dehydration. Ethanol is soluble in water as a consequence of its highly polar OH group, which readily forms hydrogen bonds with water molecules. Alkanols can be turned into amide groups by reacting ethanol with ammonia. alumina CH3CH2OH(g) + NH3(g) What type of reaction is this? 400°C CH3CH2NH2(g) + H2O(g) Alkanols can also be oxidised to form carboxylic acids: › CH3CH2OH(aq) O2 (g) CH3COOH(aq) Not all alkanols will oxidise to form carboxylic acids. Carboxylic acid synthesise only occurs from primary alkanols. Primary Secondary Tertiary Carboxylic acids are weak acids, reacting with water to form weak acidic solutions. CH3COOH(aq) + H2O(l)↔ CH3COO-(aq) + H3O+(aq) Page 151 Questions 8, 9 and 10 Esters are a group of organic compounds responsible for some of the natural and synthetic flavours and smells in ice-creams, lollies, flowers and fruits. Ester Smell or flavour Pentyl propanoate Apricot Ethyl butanoate Pineapple Octyl ethanoate Orange 2-methyl methanoate Raspberry Ethyl methanoate Rum Pentyl ethanoate Banana Esters composed of small molecules are volatile and smelly. Esters of larger molecular size are oils and waxes Esters are made by a condensation reaction between carboxylic acids and an alkanol. Reactions that involve the combination of two reactions and the elimination of a small molecule, such as water, are called condensation reactions. Gently heating a mixture of ethanol and pure ethanoic acid, with a trace amount of sulfuric acid as a catalyst, produces an ester (ethyl ethanoate) and water. Ethyl ethanoate is more commonly known as ethyl acetate, it is used as a solvent in paints and nail varnish Below is the general equation for the esterification reaction involving a carboxylic acid and an alkanol. Esters have two-part names. The first part derived from the name of the alkanol from which it is made › The ‘anol’ part is replace with ‘yl’ › Ethanol becomes ethyl The second part comes from the carboxylic acid. › Where ‘ic acid’ is replace with ‘ate’ › Ethanoic acid becomes ethanoate. Therefore we have ethyl ethanoate Read pages 153 – 155 on polyesters Page 156 Questions 11 and 12 Which pathway is the most effective to make ethanol??? Production chemists need to find the most efficient pathway for making certain materials. To do this there are certain areas to consider: › › › › › › › How readily available is the starting material The yield (how much will it produce) The purity of the final product Can they minimise any unwanted side products Can they minimise waste materials Cost How long will it take. What is ethyl propanoate made out of? Suppose we only had alkanes and alkenes on hand how could we make ethyl propanoate? Ethanol is a two carbon compound that can be synthesised directly from ethene, or from ethene via the intermediate product chloroethane. Propanoic acid is a carboxylic acid containing 3 carbon atoms. It is prepared by the oxidation of the primary alkanol propan-1-ol. This in turn can be formed by the reaction of 1chloropropane with NaOH. 1-chloropropane is formed by reacting propane with chlorine. A number of products will be formed which are separated by fractional distillation. The substitution reaction of propane is chosen rather than an addition reaction of propene because the addition of HCl to propene will result in the formation of unwanted 2-chloropropane. Having synthesised ethanol and propanoic acid we can now prepare the ester using a condensation reaction. The purity of the product needs to be evaluated. For this a lot of companies will use some of the analysis techniques looked at in first term. The yield must be taken into account, as not all of the reactants are necessarily converted to product % Yield = Actual mass of product obtained Theoretical mass of product Page 159 Question 15, 17 and 18 A technique used to separate liquids that have different boiling points. Commonly used in a laboratory to separate volatile liquids from a reaction mixture. Industrial application of fractional distillation include: › Separation of the fractions from crude oil › Production of oxygen and nitrogen by the fractional distillation of liquid air › Extraction of ethanol from water in the fermentation of sugar. The column is packed with glass beads or has glass shelves, providing a large surface area upon which the vapours condense. There is a temparature gradient up the fractionating column; the column is cooler at the top than at the bottom. Looking at our example of synthesis of the ester ethyl ethanoate. Pure ethyl ethanoate can be extracted from the reaction mixture by fractional distillation. Look at the boiling points of components in the reaction mixture Component Boiling Point CH3COOCH2CH3 57°C CH3CH2OH 78°C H2O 100°C CH3COOH 118°C The reaction mixture is heated in the distillation flask. The vapour rises up the fractionating column. The temperature at the top of the column slowing increases until it stabilises at about 57°C, which is the boiling point of ethyl ethanoate. The fraction condensing over a small range of temperature near the boiling point of ethyl ethanoate, 55°C - 59°C is collected. Page 161 Question 19 Pharmaceutical products are often developed from substances found in a plant that has been used as a traditional medicine. Aspirin is one such substance. Its origins are from a naturally occurring substance called salicin found in the leaves and bark of willow trees and in the herb medowsweet. As long ago as 400BC people have recommended ‘an infusion of willow leaves and bark to relieve aches, pains, inflammation and fever.’ The body converts salicin into salicylic acid and this is the active substance that helps to reduce fever and acts as a pain killer. Salicylic acid is more effective than salicin and by 1870 doctors were prescribing salicylic acid directly. A lot of people could not tolerate salicylic acid directly and it tasted bad. In 1897, Felix Hoffmann, synthesised an improved modification of salicylic acid. Once he had made salicylic acid he replaced the hydroxy functional group with an ester functional group to form acetylsalicylic acid. This is the compound known commercially as aspirin. To make aspirin we could add a carboxylic acid and an alcohol. This would form acetylsalicylic acid However this is a slow reaction, with a low yield, as the water formed tends to drive the reaction backwards. In an alternative reaction pathway, which is faster and produces higher yields, the ethanoic acid is replaced with ethanoic anhydride (acetic anhydride). This is the preferred pathway for aspirin synthesis The products, acetylsalicylic acid and acetic acid have to be separated and the product purified before it can be put into tablet form and packaged for sale. Although aspirin has a –COOH functional group, pure acetylsalicylic acid is not very soluble in water. Converting the carboxylic acid functional group into the sodium salt changes the molecule into an ion and makes it much more soluble. It is used in many headache and cold remedies in this form. A recent development is to make a polymer structure using a condensation reaction between salicylic acid and 1,8-octanedioic acid Pretty much a polymer of aspirin which has a number of potential advantages: › It can be used as a controlled-release pain killer because the polymer breaks down slowly › Because it is a polymer with a similar molecular structure to polyesters it can be made into thread and used to stitch cuts or wounds together. › It has the potential to be used as a plastic coating for an injured bone or joint. Page 225 Question 1